AXE Method
Another way of looking at molecular geometries is through the "AXE method" of electron counting. A in AXE represents the central atom and always has an implied subscript one; X represents the number of sigma bonds between the central and outside atoms (multiple covalent bonds—double, triple, etc.— count as one X); and E represents the number of lone electron pairs surrounding the central atom. The sum of X and E, known as the steric number, is also associated with the total number of hybridized orbitals used by valence bond theory. VSEPR uses the steric number and distribution of X's and E's to predict molecular geometric shapes.
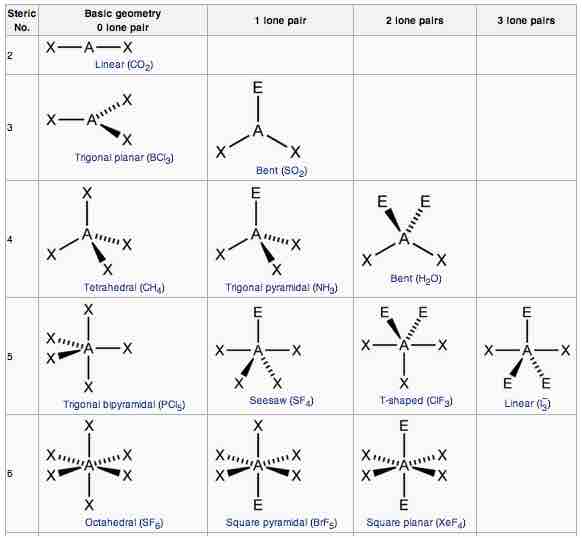
AXE method
The A represents the central atom; the X represents the number of sigma bonds between the central atoms and outside atoms; and the E represents the number of lone electron pairs surrounding the central atom. The sum of X and E, known as the steric number, is also associated with the total number of hybridized orbitals used by valence bond theory.
Note that the geometries are named according to the atomic positions only, not the electron arrangement.
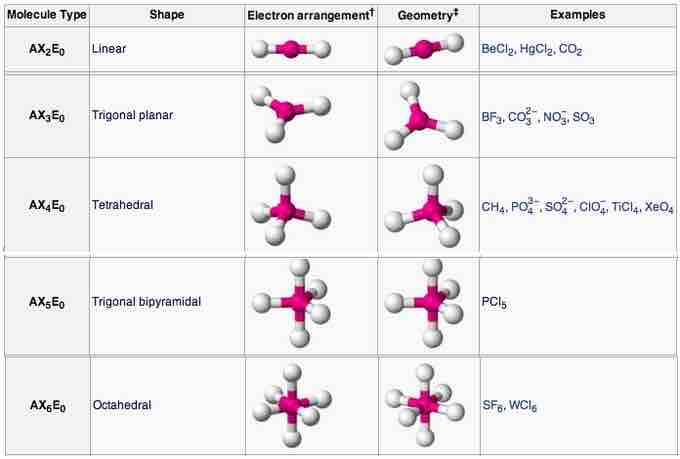
AXE method: annotation and examples
AXE annotation, geometry, and examples for each shape.
Main geometries (without lone pairs of electrons):
Linear
In a linear model, atoms are connected in a straight line, and a bond angle is simply the geometric angle between two adjacent bonds. A simple triatomic molecule of the type AX2 has its two bonding orbitals 180° apart. Examples of triatomic molecules for which VSEPR theory predicts a linear shape include BeCl2 (which does not possess enough electrons to conform to the octet rule) and CO2. When writing out the electron dot formula for carbon dioxide, notice that the C-O bonds are double bonds; this makes no difference to VSEPR theory. The central carbon atom is still joined to two other atoms. The electron clouds that connect the two oxygen atoms are 180° apart.
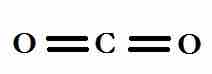
Lewis dot structure of carbon dioxide
Although the central atom (carbon) has four bonds, only two are sigma bonds; it is therefore is represented as AX2E0 in the table.
Trigonal planar
Molecules with the trigonal planar shape are triangular and in one plane, or flat surface. An AX3 molecule such as BF3 has three regions of electron density extending out from the central atom. The repulsion between these will be at a minimum when the angle between any two is 120o.
Tetrahedral
Tetra- signifies four, and -hedral relates to a face of a solid; "tetrahedral" literally means "having four faces. " This shape is found when there are four bonds all on one central atom, with no lone electron pairs. In accordance with the VSEPR theory, the bond angles between the electron bonds are 109.5o. An example of a tetrahedral molecule is methane (CH4). The four equivalent bonds point in four geometrically equivalent directions in three dimensions, corresponding to the four corners of a tetrahedron centered on the carbon atom.
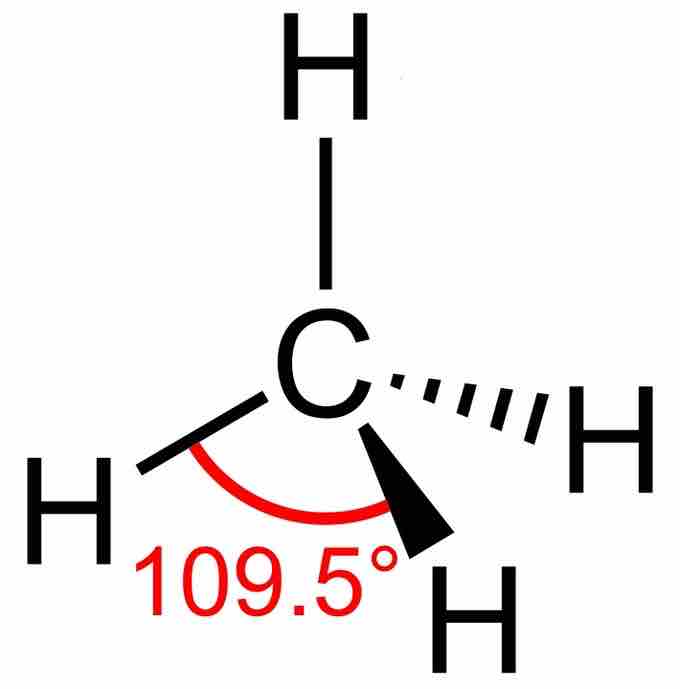
The lewis dot structure for methane
The four hydrogen atoms are equidistant from each other, with all bond angles at 109.5°.
A trigonal bipyramidal shape forms when a central atom is surrounded by five atoms in a molecule. In the geometry, three atoms are in the same plane with bond angles of 120°; the other two atoms are on opposite ends of the molecule. Some elements in Group 15 of the periodic table form compounds of the type AX5; examples include PCl5 and AsF5.
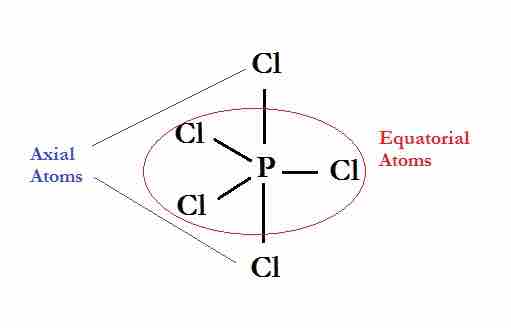
The Lewis dot structure of phosphorous pentachloride.
The three equatorial atoms are in the same plane, with the two axial atoms located on opposite ends of the molecule.
Octahedral
Octa- signifies eight, and -hedral relates to a face of a solid, so "octahedral" literally means "having eight faces." The bond angles are all 90°, and just as four electron pairs experience minimum repulsion when they are directed toward the corners of a tetrahedron, six electron pairs try to point toward the corners of an octahedron. An example of an octahedral molecule (AX6) is sulfur hexafluoride (SF6).