Concept
Version 9
Created by Boundless
Hybridization in Molecules Containing Double and Triple Bonds
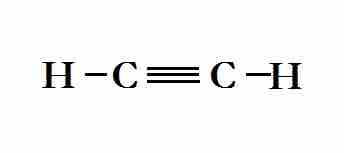
Lewis structure of ethyne, which contains a triple bond
The sp hybridized orbitals are used to overlap with the 1s hydrogen orbitals and the other carbon atom. The remaining, non-hybridized p-orbitals overlap for the double and triple pi bonds.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources: