History of Dalton's Atomic Theory
Although the concept of the atom dates back to the ideas of Democritus, the English meteorologist and chemist John Dalton formulated the first modern description of it as the fundamental building block of chemical structures. Dalton developed the law of multiple proportions (first presented in 1803) by studying and expanding upon the works of Antoine Lavoisier and Joseph Proust.
Proust had studied tin oxides and found that their masses were either 88.1% tin and 11.9% oxygen or 78.7% tin and 21.3% oxygen (these were tin(II) oxide and tin dioxide respectively). Dalton noted from these percentages that 100g of tin will combine either with 13.5g or 27g of oxygen; 13.5 and 27 form a ratio of 1:2. Dalton found an atomic theory of matter could elegantly explain this common pattern in chemistry - in the case of Proust's tin oxides, one tin atom will combine with either one or two oxygen atoms.
Dalton also believed atomic theory could explain why water absorbed different gases in different proportions: for example, he found that water absorbed carbon dioxide far better than it absorbed nitrogen. Dalton hypothesized this was due to the differences in the mass and complexity of the gases' respective particles. Indeed, carbon dioxide molecules (CO2) are heavier and larger than nitrogen molecules (N2).
Dalton proposed that each chemical element is composed of atoms of a single, unique type, and though they cannot be altered or destroyed by chemical means, they can combine to form more complex structures (chemical compounds). Since Dalton reached his conclusions by experimentation and examination of the results in an empirical fashion, this marked the first truly scientific theory of the atom.
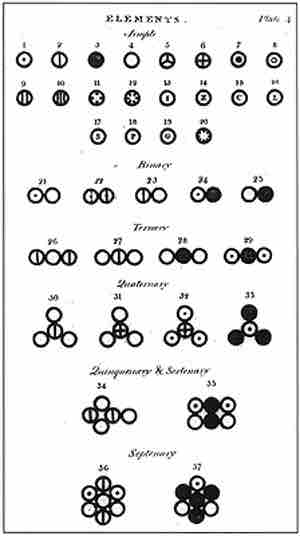
John Dalton's A New System of Chemical Philosophy
This image from Dalton's A New System of Chemical Philosophy, published in 1808, depicts various atoms and molecules.
Dalton's Atomic Theory
The main points of Dalton's atomic theory are:
- Everything is composed of atoms, which are the indivisible building blocks of matter and cannot be destroyed.
- All atoms of an element are identical.
- The atoms of different elements vary in size and mass.
- Compounds are produced through different whole-number combinations of atoms.
- A chemical reaction results in the rearrangement of atoms in the reactant and product compounds.
Atomic theory has been revised over the years to incorporate the existence of atomic isotopes and the interconversion of mass and energy. In addition, the discovery of subatomic particles has shown that atoms can be divided into smaller parts. However, Dalton's importance in the development of modern atomic theory has been recognized by the designation of the atomic mass unit as a Dalton.