Concept
Version 12
Created by Boundless
Concentration of Cells
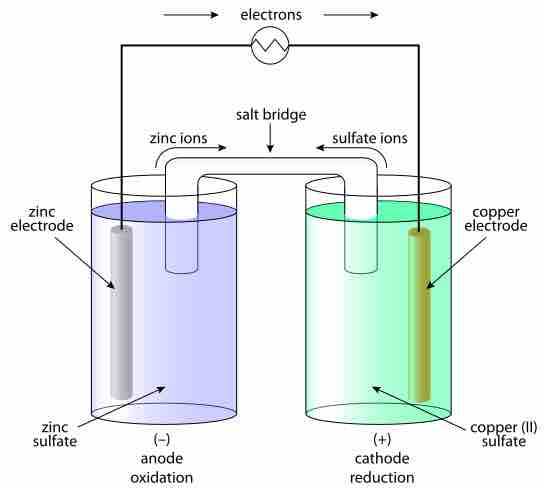
A typical galvanic electrochemical cell
Under standard conditions, the output of this pair of half-cells is well known. When a change in the concentration or activity of reactants occurs, or the temperature or pressure changes, the output voltage changes. It is calculated via the Nernst equation.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Galvanic cell labeled."
http://en.wikipedia.org/wiki/File:Galvanic_cell_labeled.svg
Wikipedia
CC BY-SA.