Concept
Version 10
Created by Boundless
Emission Spectrum of the Hydrogen Atom
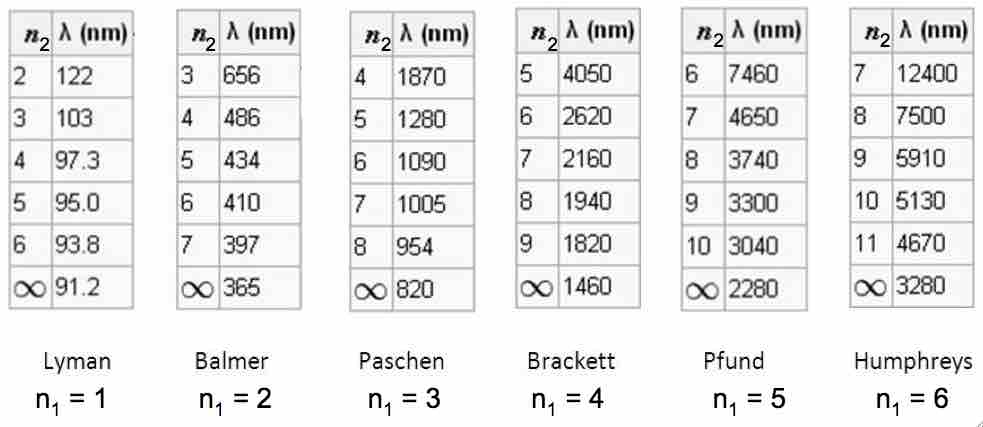
The emission spectrum of hydrogen
Some of the most common and readily observable series have been named as shown in this image, where n1 is the ground state and n2 are excited states. The various series are named for the atomic energy level they end on (n1). The series limit where n2 is infinite and n1=1 corresponds to the ionization energy of hydrogen.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Hydrogen spectral series."
http://en.wikipedia.org/wiki/Hydrogen_spectral_series
Wikipedia
CC BY-SA 3.0.