Concept
Version 11
Created by Boundless
The Pauli Exclusion Principle
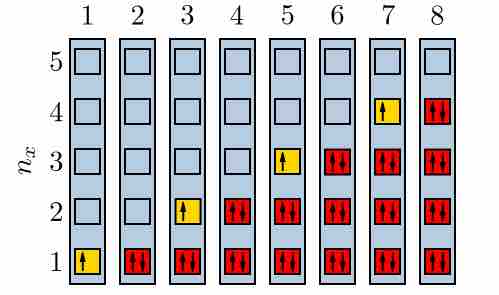
Electrons filling quantum energy levels
When a state has only one electron, it could be either spin-up or spin-down. However, according the the Pauli Exclusion Principle, when there are two in a state, there must be one of each.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Materials in Electronics/The Aufbau Principle."
http://en.wikibooks.org/wiki/Materials_in_Electronics/The_Aufbau_Principle
Wikibooks
CC BY-SA 3.0.