Compounds with Anionic Hydrogen
A hydride is the anion of hydrogen (H−), and it can form compounds in which one or more hydrogen centers have nucleophilic, reducing, or basic properties. In such hydrides, hydrogen is bonded to a more electropositive element or group.
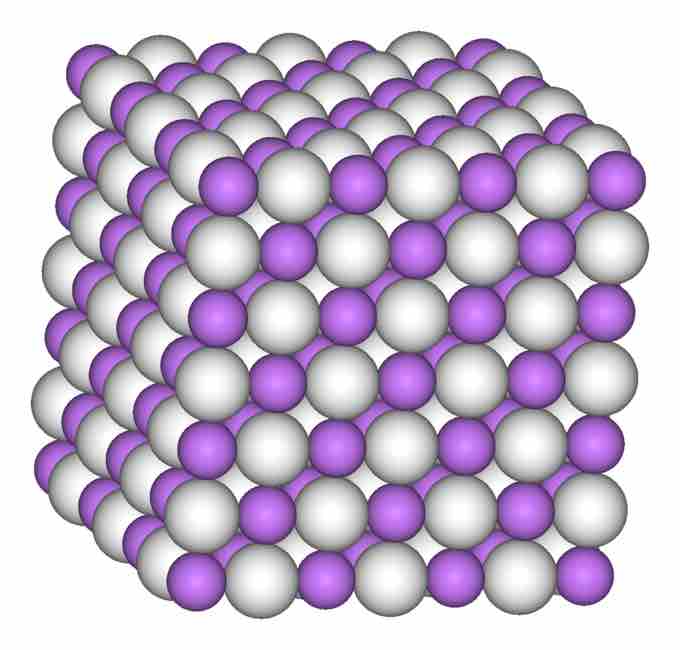
Lithium Hydride, LiH
This is a space-filling model of a crystal of lithium hydride, LiH, a binary halide.
Hydride compounds often do not conform to classical electron-counting rules, but are described as multi-centered bonds with metallic bonding. Hydrides can be components of discrete molecules, oligomers, polymers, ionic solids, chemisorbed monolayers, bulk metals (interstitial), and other materials. While hydrides traditionally react as Lewis bases or reducing agents by donating electrons, some metal hydrides behave as both acids and hydrogen-atom donors.
Applications of Hydrides
Hydrides are commonly used as reducing agents, donating electrons in chemical reactions. Hydrides can be used as strong bases in organic syntheses, and their reaction with weak Bronsted acids releases dihydrogen (H2).
Hydrides such as calcium hydride are used as dessicants, or drying agents, to remove trace water from organic solvents. In such cases, the hydride reacts with water, forming diatomic hydrogen and a hydroxide salt:
The dry solvent can then be distilled or vac-transferred from the "solvent pot."
Hydride complexes are catalysts and catalytic intermediates in a variety of homogeneous and heterogeneous catalytic cycles. Important examples include hydrogenation, hydroformylation, hydrosilylation, and hydrodesulfurization catalysts. Even certain enzymes, like hydrogenase, operate via hydride intermediates. The energy carrier NADH reacts as a hydride donor or hydride equivalent.
Free hydride anions exist only under extreme conditions and are not invoked for homogeneous solutions. Instead, many compounds have a hydrogen center with a hydridic character. Hydrides can be characterized as ionic, covalent, or interstitial hydrides based on their bonding types.
Ionic Hydrides
Ionic, or saline, hydride is a hydrogen atom bound to an extremely electropositive metal, generally an alkali metal or an alkaline earth metal (for example, potassium hydride or KH). These types of hydrides are insoluble in conventional solvents, reflecting their non-molecular structures. Most ionic hydrides exist as "binary" materials that involve only two elements, one of which is hydrogen. Ionic hydrides are often used as heterogeneous bases and reducing reagents in organic synthesis.
Covalent Hydrides
Covalent hydrides refer to hydrogen centers that react as hydrides, or those that are nucleophilic. In these substances, the hydride bond, formally, is a covalent bond much like the bond that is made by a proton in a weak acid. This category includes hydrides that exist as discrete molecules, polymers, oligomers, or hydrogen that has been chem-adsorbed to a surface. A particularly important type of covalent hydride is the complex metal hydride, a powerful (reducing) soluble hydride that is commonly used in organic syntheses (for example, sodium borohydride or NaBH4). Transition metal hydrides also include compounds that can be classified as covalent hydrides. Some are even classified as interstitial hydrides and other bridging hydrides. Classical transition metal hydrides feature a single bond between the hydrogen center and the transition metal.
Interstitial or Metallic Hydrides
Interstitial hydrides most commonly exist within metals or alloys. Their bonding is generally considered metallic. Such bulk transition metals form interstitial binary hydrides when exposed to hydrogen. These systems are usually non-stoichiometric, with variable amounts of hydrogen atoms in the lattice.