Concept
Version 14
Created by Boundless
Energy Changes in Chemical Reactions
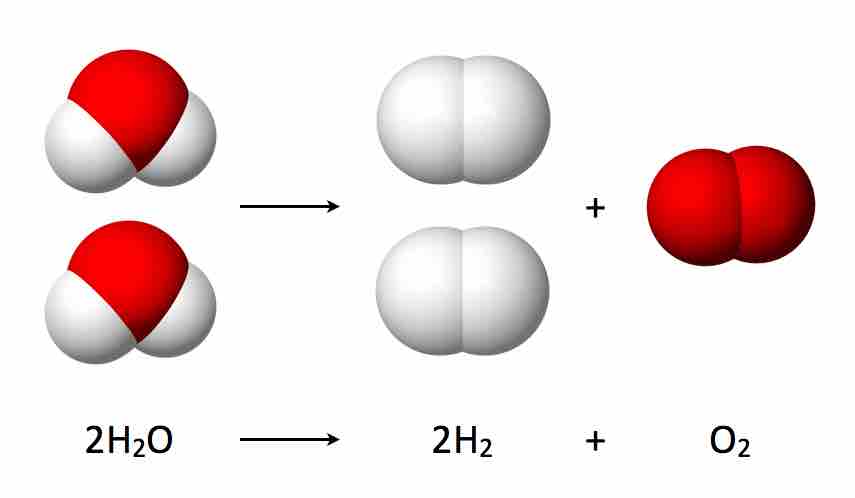
The decomposition of water into hydrogen and oxygen
When water is heated to over 2000 degrees Celsius, a small fraction will decompose into hydrogen and oxygen. Significant heat energy is needed for this reaction to proceed, so the reaction is endothermic.
2 H20 becomes 2 H2 molecules and an O2 molecule.
Source
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
"Electrolysis of Water."
http://en.wikipedia.org/wiki/Electrolysis_of_water%23mediaviewer/File:Electrolysis_of_Water.png
WikiPedia
CC BY-SA 3.0.