Isotopes
The term isotope refers to the number of neutrons a certain element contains. Elements of the same name (for example, oxygen) must always have the same number of protons, but the number of neutrons can change. Adding or subtracting neutrons from an atom does not change the elemental properties, but it can alter some of its features (like making it more radioactive).
Stable Isotopes
While the number of neutrons in a particular atom can change, there is a certain threshold where the atom is given more neutrons that its nuclear force can hold. At this point, the neutrons start to be released. The release is also known as decay . During this time, the atom is deemed "unstable. " The atom will continue to lose neutrons until it become stable again. A stable isotope is a chemical isotope that is not radioactive. There are some cases where atoms have no stable isotopes so they continue to lose neutrons, and later protons and electrons, until they become another element entirely. Research has shown that there are 80 elements with one or more stable isotopes. Of these, 26 have only one stable isotope which is also known as being monoisotopic. The element with the most stable isotopes is tin which as 10 stable isotopes.
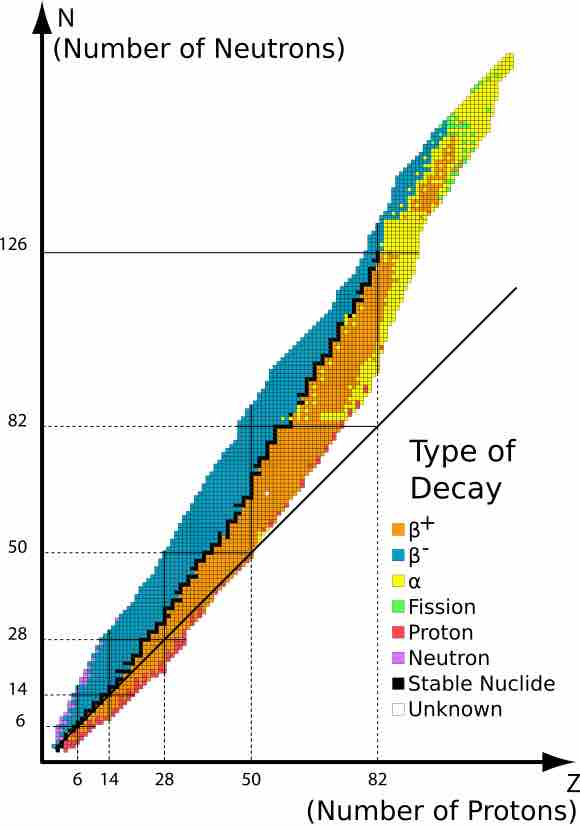
Table of Isotopes
This is a table that represents atom decay yielding various isotopes.
Extensions
Knowledge about stable isotopes is important in a variety of fields. Scientists have used information on these topics in botanical and plant biological investigations as well as ecological and biological studies. Additionally, some scientists have used oxygen isotope ratios to reconstruct historical atmospheric temperatures. This work is especially important due to our current concern with climate change/global warming.