Attenuation (in genetics) is a proposed mechanism of control in some bacterial operons that results in premature termination of transcription. It is based on the fact that, in bacteria, transcription and translation can and do proceed simultaneously. Attenuation involves a provisional stop signal (attenuator), located in the DNA segment that corresponds to the leader sequence of mRNA. During attenuation, the ribosome becomes stalled (delayed) in the attenuator region in the mRNA leader. Depending on the metabolic conditions, the attenuator either stops transcription at that point or allows read-through to the structural gene part of the mRNA and synthesis of the appropriate protein.
Attenuation is a regulatory feature found throughout Archaea and Bacteria causing premature termination of transcription. Attenuators are 5'-cis acting regulatory regions that fold into one of two alternative RNA structures that determine the success of transcription. The folding is modulated by a sensing mechanism producing either a Rho-independent terminator, resulting in interrupted transcription and a non-functional RNA product; or an anti-terminator structure, resulting in a functional RNA transcript. There are now many equivalent examples where the translation, not transcription, is terminated by sequestering the Shine-Dalgarno sequence (ribosomal binding site) in a hairpin-loop structure. While not meeting the previous definition of (transcriptional) attenuation, these are now considered to be variants of the same phenomena and are included in this article. Attenuation is an ancient regulatory system, prevalent in many bacterial species providing fast and sensitive regulation of gene operons and is commonly used to repress genes in the presence of their own product (or a downstream metabolite). What is an attenuator? Attenuator is a nucleotide sequence in DNA that can lead to premature termination of transcription.
Attenuators may be classified according to the type of molecule which induces the change in RNA structure. It is likely that transcription-attenuation mechanisms developed early, perhaps prior to the archaea/bacteria separation and have since evolved to use a number of different sensing molecules (the tryptophan biosynthetic operon has been found to use three different mechanisms in different organisms. )
An example is the trp gene in bacteria . When there is a high level of tryptophan in the region, it is inefficient for the bacterium to synthesize more. When the RNA polymerase binds and transcribes the trp gene, the ribosome will start translating. (This differs from eukaryotic cells, where RNA must exit the nucleus before translation starts. ) The attenuator sequence, which is located between the mRNA leader sequence (5' UTR) and trp operon gene sequence, contains four domains, where domain 3 can pair with domain 2 or domain 4.
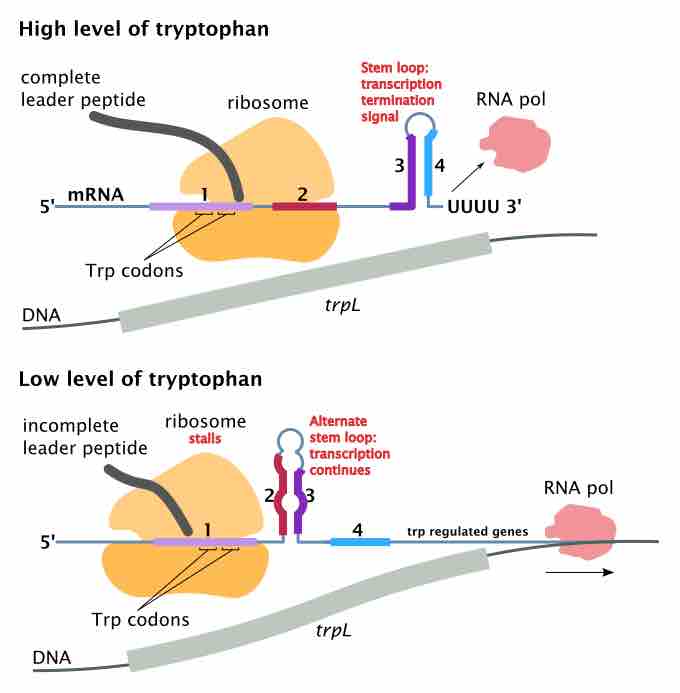
Mechanism of transcriptional attenuation of the trp operon.
Mechanism of transcriptional attenuation of the trp operon.
The attenuator sequence at domain 1 contains instruction for peptide synthesis that requires tryptophans. A high level of tryptophan will permit ribosomes to translate the attenuator sequence domains 1 and 2, allowing domains 3 and 4 to form a hairpin structure, which results in termination of transcription of the trp operon. Since the protein coding genes are not transcribed due to rho independent termination, no tryptophan is synthesized.
In contrast, a low level of tryptophan means that the ribosome will stall at domain 1, causing the domains 2 and 3 to form a different hairpin structure that does not signal termination of transcription. Therefore, the rest of the operon will be transcribed and translated, so that tryptophan can be produced. Thus, domain 4 is an attenuator. Without domain 4, translation can continue regardless of the level of tryptophan. The attenuator sequence has its codons translated into a leader peptide, but is not part of the trp operon gene sequence. The attenuator allows more time for the attenuator sequence domains to form loop structures, but does not produce a protein that is used in later tryptophan synthesis.