Nitrogen fixation also refers to other biological conversions of nitrogen, such as its conversion to nitrogen dioxide. Nitrogen fixation is a process by which nitrogen (N2) in the atmosphere is converted into ammonia (NH3). Atmospheric nitrogen or elemental nitrogen (N2) is relatively inert: it does not easily react with other chemicals to form new compounds. Dinitrogen is quite inert because of the strength of its N≡N triple bond. To break one nitrogen atom away from another requires breaking all three of these chemical bonds. Fixation processes free up the nitrogen atoms from their diatomic form (N2) to be used in other ways. Nitrogen fixation, natural and synthetic, is essential for all forms of life because nitrogen is required to biosynthesize basic building blocks of plants, animals, and other life forms, e.g., nucleotides for DNA and RNA and amino acids for proteins. Therefore, nitrogen fixation is essential for agriculture and the manufacture of fertilizer. Microorganisms that fix nitrogen are bacteria called diazotrophs.
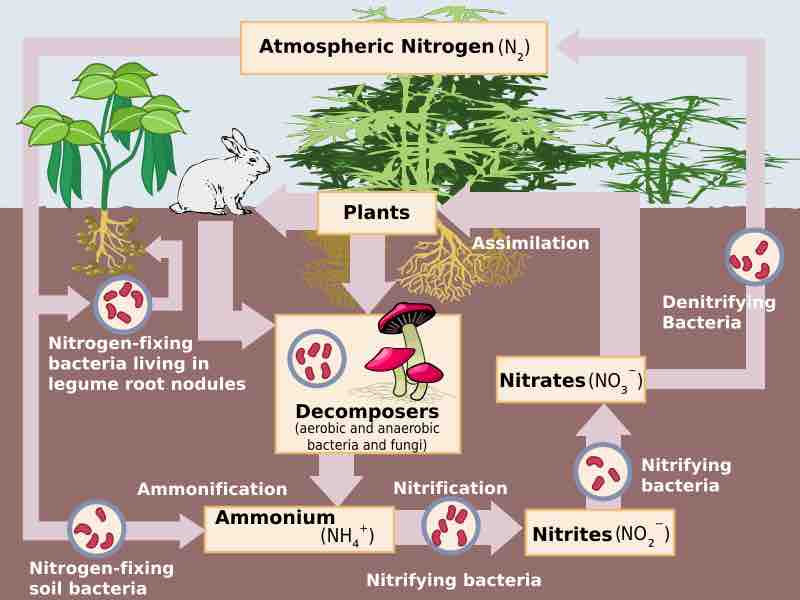
The role of soil bacteria in the Nitrogen cycle
Nitrogen transitions between various biologically useful forms.
Some higher plants, and some animals (termites), have formed associations (symbioses) with diazotrophs. Diazotrophs are microbes. They intensively studied by microbiologists. Biological nitrogen fixation was discovered by the German agronomist Hermann Hellriegel and Dutch microbiologist Martinus Beijerinck. Biological nitrogen fixation (BNF) occurs when atmospheric nitrogen is converted to ammonia by an enzyme called nitrogenase. Nitrogenases are enzymes used by some organisms to fix atmospheric nitrogen gas (N2). There is only one known family of enzymes that accomplishes this process. All nitrogenases have an iron- and sulfur-containing cofactor that includes a heterometal complex in the active site (e.g., FeMoCo). In most species, this heterometal complex has a central molybdenum atom. However, in some species it is replaced by a vanadium or iron atom. Enzymes responsible for nitrogenase action are very susceptible to destruction by oxygen. Many bacteria cease production of the enzyme in the presence of oxygen. Many nitrogen-fixing organisms exist only in anaerobic conditions, respiring to draw down oxygen levels, or binding the oxygen with protins.