A periodic table is a tabular display of the chemical elements, organized on the basis of their atomic numbers, electron configurations, and recurring chemical properties. Elements are presented according to their atomic numbers (number of protons) in increasing order. The standard form of the table comprises an eighteen by seven grid or main body of elements, positioned above a smaller double row of elements. The table can also be deconstructed into four rectangular blocks: the s-block to the left, the p-block to the right, the d-block in the middle, and the f-block below that. The rows of the table are called periods. The columns of the s-, d-, and p-blocks are called groups, some of which have names such as the halogens or the noble gases.
Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new elements that are yet to be discovered or synthesized. As a result, a periodic table, in the standard form or some other variant, provides a useful framework for analyzing chemical behavior. Such tables are widely used in chemistry and other sciences.
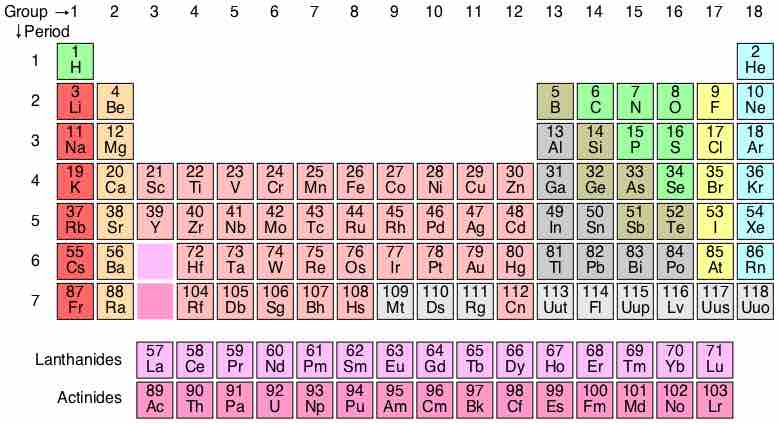
Periodic Table of Elements
The standard form of the periodic table, where the colors represent different categories of elements
The Specifics of the Periodic Table
All versions of the periodic table include only chemical elements, rather than mixtures, compounds, or subatomic particles. Each chemical element has a unique atomic number representing the number of protons in its nucleus. Most elements have differing numbers of neutrons among different atoms: these variants are referred to as isotopes. For example, carbon has three naturally occurring isotopes. All of its atoms have six protons and most have six neutrons as well, but about one percent have seven neutrons, and a very small fraction have eight neutrons. Isotopes are never separated in the periodic table. They are always grouped together under a single element. Elements with no stable isotopes have the atomic masses of their most stable isotopes listed in parentheses.
All elements from atomic numbers '1' (hydrogen) to '118' (ununoctium) have been discovered or synthesized. Of these, elements up through californium exist naturally; the rest have only been synthesized in laboratories. The production of elements beyond ununoctium is being pursued. The question of how the periodic table may need to be modified to accommodate any such additions is a matter of ongoing debate. Numerous synthetic radionuclides of naturally occurring elements have also been produced in laboratories.
Although precursors exist, Dmitri Mendeleev is generally credited with the publication of the first widely recognized periodic table in 1869. He developed his table to illustrate periodic trends in the properties of the elements known at the time. Mendeleev also predicted some properties of then-unknown elements that were expected to fill gaps in the table. Most of his predictions were proved correct when the elements in question were subsequently discovered. Mendeleev's periodic table has since been expanded and refined with the discovery or synthesis of more new elements and the development of new theoretical models to explain chemical behavior.
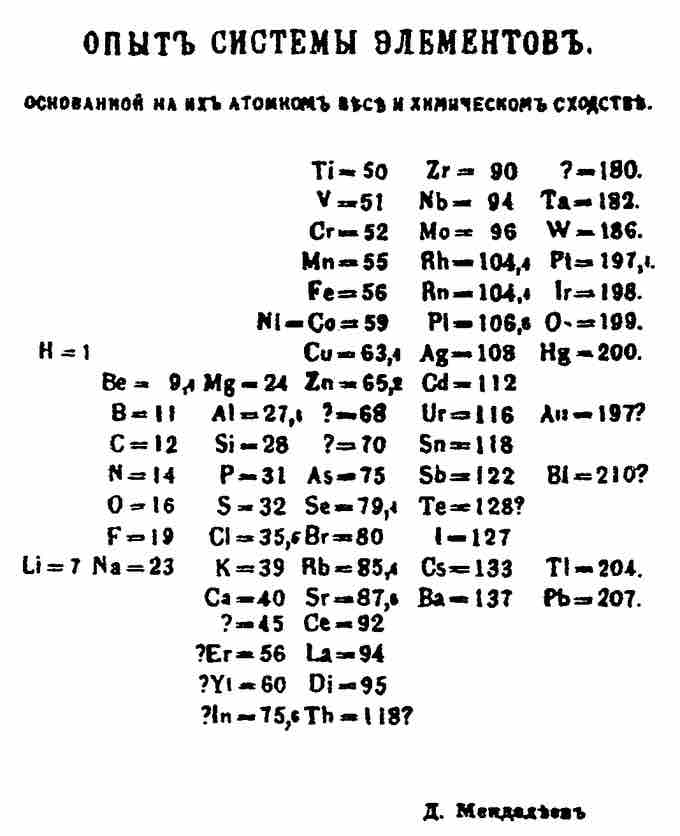
Mendeleev's 1869 Periodic Table
Mendeleev's 1869 periodic table presents the periods vertically and the groups horizontally.

Dmitri Mendeleev
Dmitri Mendeleev is known for publishing a widely recognized periodic table.