The periodic table is a tabular display of the chemical elements. The elements are organized based on their atomic numbers, electron configurations, and recurring chemical properties.
In the periodic table, elements are presented in order of increasing atomic number (the number of protons). The rows of the table are called periods; the columns of the s- (columns 1-2 and He), d- (columns 3-12), and p-blocks (columns 13-18, except He) are called groups . (The terminology of s-, p-, and d- blocks originate from the valence atomic orbitals the element's electrons occupy. ) Some groups have specific names, such as the halogens or the noble gases. Since, by definition, a periodic table incorporates recurring trends, any such table can be used to derive relationships between the properties of the elements and predict the properties of new, yet-to-be-discovered, or synthesized elements. As a result, the periodic table provides a useful framework for analyzing chemical behavior, and such tables are widely used in chemistry and other sciences.
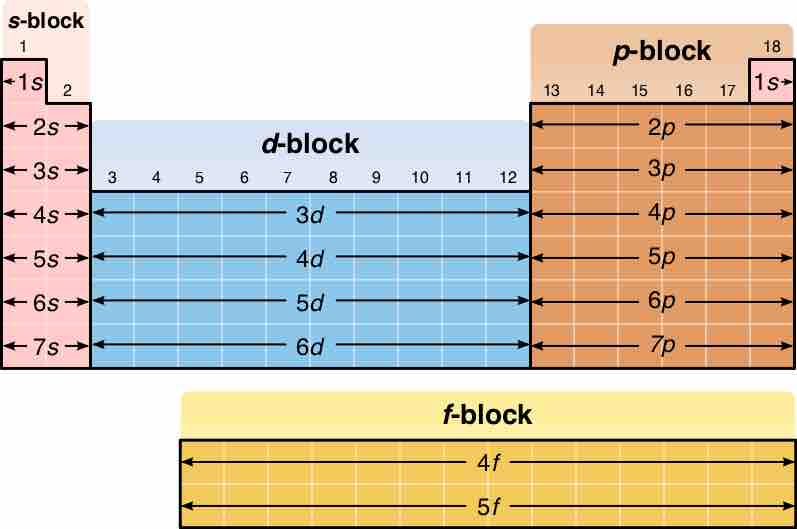
Blocks in the Periodic Table
A diagram of the periodic table, highlighting the different blocks
History of the Periodic Table
Although precursors exist, Dmitri Mendeleev is generally credited with the publication, in 1869, of the first widely recognized periodic table. Mendeleev designed the table in such a way that recurring ("periodic") trends in the properties of the elements could be shown. Using the trends he observed, he even left gaps for those elements that he thought were "missing. " He even predicted the properties that he thought the missing elements would have when they were discovered. Many of these elements were indeed later discovered, and Mendeleev's predictions were proved to be correct.
Groups
Agroup, or family, is a vertical column in the periodic table. Groups usually have more significant periodic trends than do periods and blocks, which are explained below. Modern quantum mechanical theories of atomic structure explain group trends by proposing that elements in the same group generally have the same electron configurations in their valence (or outermost, partially filled) shell. Consequently, elements in the same group tend to have shared chemistry and exhibit a clear trend in properties with increasing atomic number. However, in some parts of the periodic table, such as the d-block and the f-block, horizontal similarities can be as important as, or more pronounced than, vertical similarities.
Periods
A period is a horizontal row in the periodic table. Although groups generally have more significant periodic trends, there are regions where horizontal trends are more significant than vertical group trends, such as in the f-block, where the lanthanides and actinides form two substantial horizontal series of elements. Elements in the same period show trends in atomic radius, ionization energy, and electron affinity. Atomic radius usually decreases from left to right across a period. This occurs because each successive element has an added proton and electron, which causes the electron to be drawn closer to the nucleus, decreasing the radius.
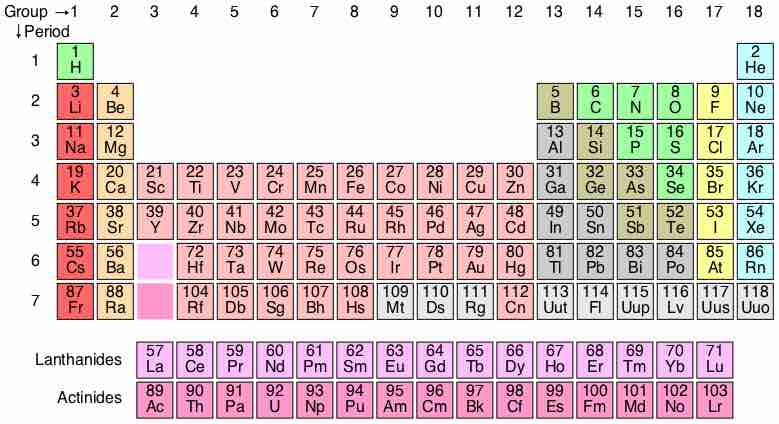
The periodic table
Here is the complete periodic table with atomic numbers, groups, and periods. Each entry on the periodic table represents one element, and compounds are made up of several of these elements.