Absolute zerois the coldest possible temperature. Formally, it is the temperature at which entropy reaches its minimum value. More simply put, absolute zero refers to a state in which all the energy of a system is extracted (by definition, the lowest energy state the system can have). Absolute zero is universal in the sense that all matteris in ground state at this temperature . Therefore, it is a natural choice as the null point for a temperature unit system.
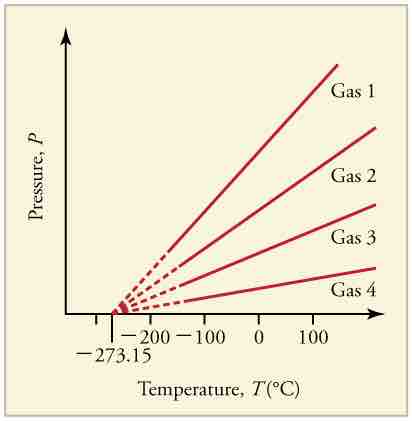
Graph of Pressure Versus Temperature
Graph of pressure versus temperature for various gases kept at a constant volume. Note that all of the graphs extrapolate to zero pressure at the same temperature
To be precise, a system at absolute zero still possesses quantum mechanical zero-point energy, the energy of its ground state. The uncertainty principle states that the position of a particle cannot be determined with absolute precision; therefore a particle is in motion even if it is at absolute zero, and a ground state still carries a minimal amount of kinetic energy. However, in the interpretation of classical thermodynamics, kinetic energy can be zero, and the thermal energy of matter vanishes.
The zero point of a thermodynamic temperature scale, such as the Kelvin scale, is set at absolute zero. By international agreement, absolute zero is defined as 0K on the Kelvin scale and as -273.15° on the Celsius scale (equivalent to -459.67° on the Fahrenheit scale). Scientists have brought systems to temperatures very close to absolute zero, at which point matter exhibits quantum effects such as superconductivity and superfluidity. The lowest temperature that has been achieved in the laboratory is in the 100 pK range, where pK (pico-Kelvin) is equivalent to 10-12 K. The lowest natural temperature ever recorded is approximately 1K, observed in the rapid expansion of gases leaving the Boomerang Nebula, shown below .

Boomerang Nebula
The rapid expansion of gases resulting in the Boomerang Nebula causes the lowest observed temperature outside a laboratory.