Now that we understand that chemical reactions occur with a simultaneous change in energy, we can apply the concept more broadly. To start, remember that some chemical reactions are rather difficult to perform. For example, consider the combustion of carbon to make carbon monoxide:
2C(s) + O2(g) → 2CO(g) ΔH = ?In reality, this is extremely difficult to do; given the opportunity, carbon will react to make another compound, carbon dioxide:
2C(s) + O2(g) → 2CO2(g) ΔH = −393.5 kJIs there a way around this? Yes. It comes from the understanding that chemical equations can be treated like algebraic equations, with the arrow acting like the equals sign. Like algebraic equations, chemical equations can be combined, and if the same substance appears on both sides of the arrow, it can be canceled out (much like a spectator ion in ionic equations). For example, consider these two reactions:
2C(s) + 2O2(g) → 2CO2(g) 2CO2(g) → 2CO(g) + O2(g)If we added these two equations by combining all the reactants together and all the products together, we would get
2C(s) + 2O2(g) + 2CO2(g) → 2CO2(g) + 2CO(g) + O2(g)We note that 2CO2(g) appears on both sides of the arrow, so they cancel:
We also note that there are 2 mol of O2 on the reactant side, and 1 mol of O2 on the product side. We can cancel 1 mol of O2 from both sides:
What do we have left?
2C(s) + O2(g) → 2CO(g)This is the reaction we are looking for! So by algebraically combining chemical equations, we can generate new chemical equations that may not be feasible to perform.
What about the enthalpy changes? Hess’s lawWhen chemical equations are combined algebraically, their enthalpies can be combined in exactly the same way. states that when chemical equations are combined algebraically, their enthalpies can be combined in exactly the same way. Two corollaries immediately present themselves:
What are the equations being combined? The first chemical equation is the combustion of C, which produces CO2:
2C(s) + 2O2(g) → 2CO2(g)This reaction is two times the reaction to make CO2 from C(s) and O2(g), whose enthalpy change is known:
C(s) + O2(g) → CO2(g) ΔH = −393.5 kJAccording to the first corollary, the first reaction has an energy change of two times −393.5 kJ, or −787.0 kJ:
2C(s) + 2O2(g) → 2CO2(g) ΔH = −787.0 kJThe second reaction in the combination is related to the combustion of CO(g):
2CO(g) + O2(g) → 2CO2(g) ΔH = −566.0 kJThe second reaction in our combination is the reverse of the combustion of CO. When we reverse the reaction, we change the sign on the ΔH:
2CO2(g) → 2CO(g) + O2(g) ΔH = +566.0 kJNow that we have identified the enthalpy changes of the two component chemical equations, we can combine the ΔH values and add them:

Hess’s law is very powerful. It allows us to combine equations to generate new chemical reactions whose enthalpy changes can be calculated, rather than directly measured.
Determine the enthalpy change of
C2H4 + 3O2 → 2CO2 + 2H2O ΔH = ?from these reactions:
C2H2 + H2 → C2H4 ΔH = −174.5 kJ 2C2H2 + 5O2 → 4CO2 + 2H2O ΔH = −1,692.2 kJ 2CO2 + H2 → 2O2 + C2H2 ΔH = −167.5 kJSolution
We will start by writing chemical reactions that put the correct number of moles of the correct substance on the proper side. For example, our desired reaction has C2H4 as a reactant, and only one reaction from our data has C2H4. However, it has C2H4 as a product. To make it a reactant, we need to reverse the reaction, changing the sign on the ΔH:
C2H4 → C2H2 + H2 ΔH = +174.5 kJWe need CO2 and H2O as products. The second reaction has them on the proper side, so let us include one of these reactions (with the hope that the coefficients will work out when all our reactions are added):
2C2H2 + 5O2 → 4CO2 + 2H2O ΔH = −1,692.2 kJWe note that we now have 4 mol of CO2 as products; we need to get rid of 2 mol of CO2. The last reaction has 2CO2 as a reactant. Let us use it as written:
2CO2 + H2 → 2O2 + C2H2 ΔH = −167.5 kJWe combine these three reactions, modified as stated:
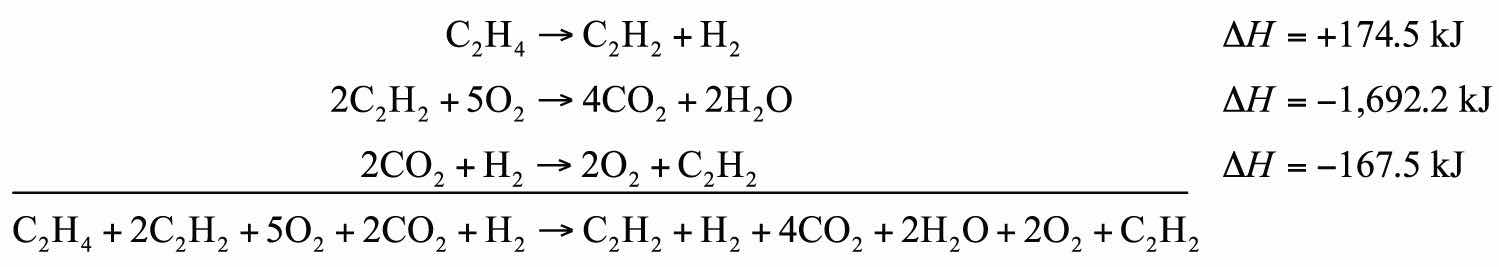
What cancels? 2C2H2, H2, 2O2, and 2CO2. What is left is
C2H4 + 3O2 → 2CO2 + 2H2Owhich is the reaction we are looking for. The ΔH of this reaction is the sum of the three ΔH values:
ΔH = +174.5 − 1,692.2 − 167.5 = −1,685.2 kJTest Yourself
Given the thermochemical equations
Pb + Cl2 → PbCl2 ΔH = −223 kJ PbCl2 + Cl2 → PbCl4 ΔH = −87 kJdetermine ΔH for
2PbCl2 → Pb + PbCl4Answer
+136 kJ
Define Hess’s law.
What does Hess’s law require us to do to the ΔH of a thermochemical equation if we reverse the equation?
If the ΔH for
C2H4 + H2 → C2H6is −65.6 kJ, what is the ΔH for this reaction?
C2H6 → C2H4 + H2If the ΔH for
2Na + Cl2 → 2NaClis −772 kJ, what is the ΔH for this reaction:
2NaCl → 2Na + Cl2If the ΔH for
C2H4 + H2 → C2H6is −65.6 kJ, what is the ΔH for this reaction?
2C2H4 + 2H2 → 2C2H6If the ΔH for
2C2H6 + 7O2 → 4CO2 + 6H2Ois −2,650 kJ, what is the ΔH for this reaction?
6C2H6 + 21O2 → 12CO2 + 18H2OThe ΔH for
C2H4 + H2O → C2H5OHis −44 kJ. What is the ΔH for this reaction?
2C2H5OH → 2C2H4 + 2H2OThe ΔH for
N2 + O2 → 2NOis 181 kJ. What is the ΔH for this reaction?
NO → 1/2N2 + 1/2O2Determine the ΔH for the reaction
Cu + Cl2 → CuCl2given these data:
2Cu + Cl2 → 2CuCl ΔH = −274 kJ 2CuCl + Cl2 → 2CuCl2 ΔH = −166 kJDetermine ΔH for the reaction
2CH4 → 2H2 + C2H4given these data:
CH4 + 2O2 → CO2 + 2H2O ΔH = −891 kJ C2H4 + 3O2 → 2CO2 + 2H2O ΔH = −1,411 kJ 2H2 + O2 → 2H2O ΔH = −571 kJDetermine ΔH for the reaction
Fe2(SO4)3 → Fe2O3 + 3SO3given these data:
4Fe + 3O2 → 2Fe2O3 ΔH = −1,650 kJ 2S + 3O2 → 2SO3 ΔH = −792 kJ 2Fe + 3S + 6O2 → Fe2(SO4)3 ΔH = −2,583 kJDetermine ΔH for the reaction
CaCO3 → CaO + CO2given these data:
2Ca + 2C + 3O2 → 2CaCO3 ΔH = −2,414 kJ C + O2 → CO2 ΔH = −393.5 kJ 2Ca + O2 → 2CaO ΔH = −1,270 kJIf chemical equations are combined, their energy changes are also combined.
ΔH = 65.6 kJ
ΔH = −131.2 kJ
ΔH = 88 kJ
ΔH = −220 kJ
ΔH = 570 kJ