Pain Management Medications
- Article Author:
- Daniel Queremel Milani
- Article Editor:
- Donald Davis
- Updated:
- 7/8/2020 2:26:44 PM
- For CME on this topic:
- Pain Management Medications CME
- PubMed Link:
- Pain Management Medications
Indications
According to the International Association for the Study of Pain (IASP), pain is defined as an unpleasant experience (sensory and/or emotional) related to a potential or confirmed tissue damage or described in such terms.[1] Currently, the debate is ongoing whether or not this definition should be modified.[2][3][4] Nonetheless, the classification for pain management medications is stable; categories are nonopioid analgesic agents and opioid analgesic agents.
Nonopioid analgesic agents
- Acetaminophen (paracetamol): Mild-to-moderate pain, moderate to severe pain (as adjunctive therapy to opioids), and temporary reduction of fever. Acetaminophen should not be used for neuropathic pain since there is no documented effect.[5][6][7]
- Nonsteroidal anti-inflammatory drugs (NSAIDs): These drugs are used for mild-to-moderate pain, pain associated with inflammation, and temporary reduction of fever. Similar to the previous medication, NSAIDs have no evidence for the management of neuropathic pain. Some NSAIDs have other non-pain related indications (eg., aspirin use for secondary prevention of myocardial infarction), which will not be covered in this review.[7][8]
- Antidepressant medications: Selective serotonin and norepinephrine reuptake inhibitors (SNRIs), particularly duloxetine, and tricyclic antidepressants (TCAs), especially amitriptyline, have demonstrated efficacy in a variety of neuropathic pain conditions. Thus they are recommended as the first line of treatment.[9] Furthermore, besides their respective indications for psychiatric disorders such as major depressive disorder and generalized anxiety disorder, these medications are indicated for other pathologies such as fibromyalgia and chronic musculoskeletal pain. Also, antidepressants are recommended as a prophylactic treatment for migraine and tension-type headache (amitriptyline). Both of the pharmacological groups seem to be more effective in patients with depressive symptoms and pain as comorbidity than in those patients with pain alone.[7][9]
- Antiepileptic medications: Several antiepileptic drugs are also known for their analgesic properties through their mechanism of action of lowering neurotransmitter release or neuronal firing. The most common antiepileptics used for pain treatment are gabapentin and pregabalin.[7]
- Gabapentin: Postherpetic neuralgia in adults and neuropathic pain.
- Pregabalin: Neuropathic pain associated with diabetic peripheral neuropathy or spinal cord injury, postherpetic neuralgia, and fibromyalgia.
- Oxcarbazepine and carbamazepine: trigeminal or glossopharyngeal neuralgia
- Local anesthetics: Lidocaine is among the most commonly used medications in this drug class, which is FDA approved for postherpetic neuralgia and recommended for peripheral neuropathic pain.[7][10]
Opioid agents
Opioids are a broad class of medications with structural resemblance to the natural plant alkaloids found in opium, which was originally derived from the resin of the opium poppy, Papaver somniferum.[11] They are recognized as the most effective and widely used drugs in treating severe pain.[12] Opioids have been among the most controversial analgesics, particularly because of their potential for addiction, tolerance, and side effects.[13] Although opioids have indications for acute and chronic pain treatment, the Center for Disease Control and Prevention's guidelines recommends that only if the expected benefits for both pain and function outweigh the risks, clinicians should prescribe opioids at the lowest effective dose and for the shortest expected duration to treat the pain severe enough to require opioids.[14][15][16][17]
Mechanism of Action
Nonopioid analgesic agents
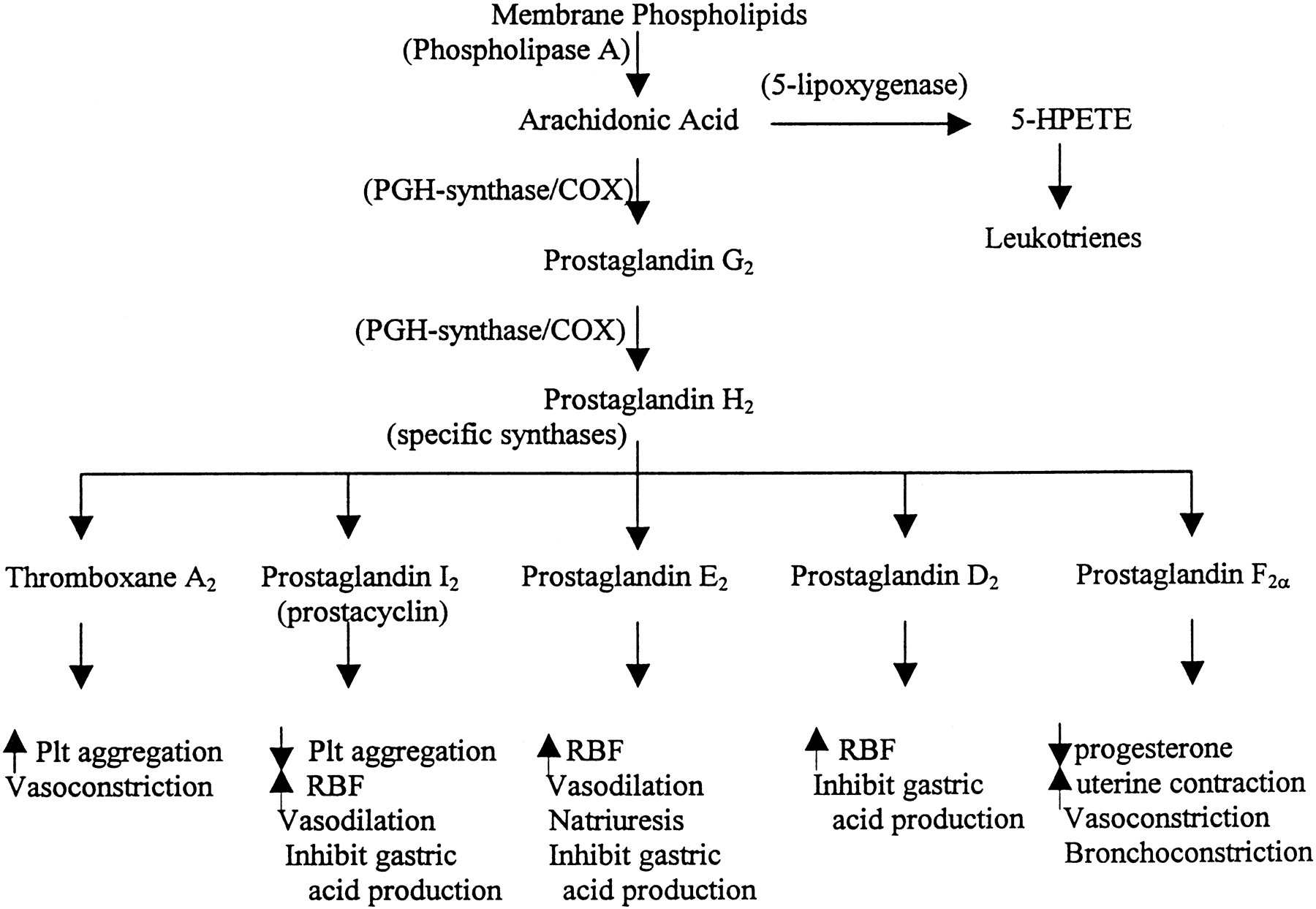
- Acetaminophen (paracetamol): The precise mechanism of action for this drug remains unclear to date. Although it is considered for some as a nonsteroidal anti-inflammatory drug (NSAID), acetaminophen lacks anti-inflammatory properties and does not bind to the active site of either cyclooxygenase (COX) enzymes (COX-1 or COX-2). However, there is a hypothesis that acetaminophen inhibits a different variant of COX-1, also known as COX-3, but this event remains unconfirmed in human studies. Nonetheless, the diminished activity of the COX pathway leads to decreased prostaglandin synthesis in the central nervous system, thus inducing analgesia (serotonergic inhibitory pathways) and antipyresis (hypothalamic heat-regulating center).[5][6][18][19]
- Nonsteroidal anti-inflammatory drugs (NSAIDs): The primary mechanism of action is the inhibition of the cyclooxygenase enzyme, thereby inhibiting prostaglandin synthesis. Drugs in this group are categorized according to chemical structure and selectivity: acetylated salicylates (aspirin), non-acetylated salicylates (diflunisal), propionic acids (ibuprofen, naproxen), acetic acids (indomethacin, diclofenac), anthranilic acids (meclofenamate, mefenamic acid), enolic acids (meloxicam, piroxicam), naphthylalanine (nabumetone), and selective COX-2 inhibitors (celecoxib, etoricoxib). Most NSAIDs inhibit both COX isoforms (COX-1 and COX-2) with little selectivity. However, those that do bind with higher affinity to one or another (eg., aspirin, and the coxibs) will exert anti-inflammatory, analgesic, and antipyretic effects at different degrees. For this reason, low doses of aspirin manifest an antiplatelet effect, while high doses exhibit an analgesic effect.[8][20][21]
- Antidepressant medications: Both tricyclic antidepressants (TCAs) and selective serotonin and norepinephrine reuptake inhibitors (SNRIs) inhibit the reuptake of two important neurotransmitters: serotonin and noradrenaline. This inhibition increases the descending inhibitory pathways of the central nervous system related to pain. Additionally, TCAs also act on cholinergic, histamine, beta2 adrenergic, opioid, and N-methyl-D- aspartate (NMDA) receptors, and sodium channels.[7][9][22]
- Antiepileptic medications: Both gabapentin and pregabalin are ligands to the αδ subunit of the voltage-dependent calcium channels, which overexpress in patients with neuropathic pain. By reducing this calcium-dependent release of excitatory neurotransmitters, these drugs decrease neuronal excitability.[7][23]
- Local anesthetics: As other local anesthetics, lidocaine stabilizes the neuronal membrane by inhibiting sodium ion channels on the internal surface of nerve cell membranes. Thus, pain conduction through nerve impulses becomes impaired at the site of action, which contributes to the absence of systemic effects.[24]
Opioid agents
The majority of the clinically relevant opioids act primarily at the “mu receptors” and thus are considered “mu agonists.”[25] Nonetheless, opioids may also act on other receptors: kappa, delta, and sigma (all of them, including mu, are G protein-coupled receptors). Depending on which receptor is activated, different physiologic effects occur (i.e., spinal and supraspinal analgesia). Opioids exert their effects at both presynaptic and postsynaptic neurons. Presynaptically, opioids block calcium channels on nociceptive afferent nerves, thus inhibiting the release of neurotransmitters such as substance P and glutamate. Postsynaptically, opioids enhance the activity of potassium channels, thus hyperpolarizing cell membranes and increasing the required action potential to generate nociceptive neurotransmission.[12][17]
Administration
Nonopioid analgesic agents
- Acetaminophen (paracetamol): The recommended dose for adults is 650 mg to 1000 mg every 4 to 6 hours, a maximum of 4 grams/day. In children, the recommended dose is 15 mg/kg every 6 hours, up to 60 mg/kg/day.[26] Acetaminophen is available to be administered orally (tablet, capsule, syrup, oral solution, or suspension), rectally (rectal suppository), or intravenously.[6]
- Nonsteroidal anti-inflammatory drugs (NSAIDs): Currently, more than 20 different NSAIDs are commercially available. The choice of the agent depends upon several factors (eg., comorbidities, risk of bleeding). Also, the response to different NSAIDs vary between patients, and the mechanisms for these distinct responses are only partially understood. Thus, doses depend on the specific drug, and the recommendation is to prescribe the lowest effective dose for each patient for the shortest period. Listed below are various commonly known NSAIDs with their respective doses for analgesia and anti-inflammation:
- Aspirin (acetylsalicylic acid): Is dosed 325 to 650 mg every 4 to 6 hours. The maximum dose is 4000 mg/day. Aspirin is available for oral (caplet, capsule, tablet) or rectal (suppository) administration.
- Diclofenac: 50 mg every 8 hours. The maximum daily dose is 150 mg. Diclofenac is available for oral (tablet, capsule, packet), intravenous, topical (cream, gel, patch, solution), or ophthalmic administration.
- Ibuprofen: It is dosed 400 mg every 4 to 6 hours. The maximum daily dose is 3200 mg (acute) or 2400 mg (chronic). Ibuprofen is available for oral (capsule, tablet, suspension) or intravenous administration.
- Indomethacin: Immediate release: 25 to 50 mg every 8 to 12 hours. Controlled release: 75 mg once or twice daily. The maximum dose is 150 mg/day. Indomethacin is available for oral (capsule, suspension), intravenous, or rectal (suppository) administration.
- Meloxicam: Dosing is 7.5 to 15 mg once daily. The maximum daily dose is 15 mg. Meloxicam is available for oral (tablet, capsule, suspension) or intravenous administration.
- Naproxen: 250 to 500 mg every 12 hours (naproxen base) or 275 to 550 mg every 12 hours (naproxen sodium). The maximum daily dose is 1250 mg acute or 1000 mg chronic for naproxen base and 1375 mg acute or 1100 mg chronic for naproxen sodium. Naproxen is available for oral (capsule, suspension, tablet) administration only.
- Celecoxib: 200 mg daily or 100 mg every 12 hours. The maximum dose is 400 mg/day. Celecoxib is available for oral (capsule) administration only.[5][6][27]
- Antidepressant medications: Among the tricyclic antidepressants and selective serotonin and norepinephrine reuptake inhibitors, amitriptyline and duloxetine have the best-documented analgesic effects, respectively.
- Amitriptyline: Dosing is 25 to 150 mg orally (tablet) once daily or in two divided doses. The maximum single and daily doses are 75 mg and 150 mg, respectively. Caution must be taken in patients 65 years old or older with maximum daily doses above 75 mg.
- Duloxetine: Dosing is 60 to 120 mg orally (capsule) once daily or in two divided doses. The maximum daily dose is 120 mg.[7][28]
- Antiepileptic medications:
- Local anesthetics:
- Lidocaine: As a patch, lidocaine is available at a concentration of 1.8% or 5%. The recommendation is to apply 1-3 patches to intact skin for up to 12 hours/day. Other commercially available presentations include topical solutions, creams, gels, ointment, and lotions.[7]
Opioid agents
Opioids are available in diverse dosage forms to use for several routes of administration: oral, transdermal, intramuscular, intravenous, subcutaneous infusion, rectal, epidural, intrathecal, intranasal, and transmucosal.[17][30] The rationale for each route of administration, dosage range, and the dosage form is dependent on a number of factors. For more detailed information, please review the American Pain Society guidelines.
Adverse Effects
Nonopioid analgesic agents
- Acetaminophen (paracetamol): This drug is a safe and effective medication when used correctly. Documented adverse effects depend on the route of administration. If administered orally or rectally, acetaminophen may cause any of the following:
- Rash or hypersensitivity reactions (toxic epidermal necrolysis, acute generalized exanthematous pustulosis, and Stevens-Johnson syndrome)
- Hematological: anemia, leukopenia, neutropenia, pancytopenia
- Nephrotoxicity
- Metabolic and electrolyte disorders
If administered intravenously, adverse effects include nausea, vomiting, pruritus, constipation, and abdominal pain.[6] For pediatric patients, regardless of the route of administration, the most common adverse reactions are nausea, vomiting, agitation, constipation, pruritus, and atelectasis.
- Nonsteroidal anti-inflammatory drugs (NSAIDs):
- Gastrointestinal: Nausea, anorexia, dyspepsia, abdominal pain, ulcers, gastrointestinal hemorrhage, perforation, constipation, diarrhea
- Cardiovascular: Hypertension, decreased effectiveness of anti-hypertensive medications, myocardial infarction, stroke, and thromboembolic events (last three with selective COX-2 inhibitors); inhibit platelet activation, propensity for bruising and hemorrhage.
- Renal: Salt and water retention, deterioration of kidney function, edema, decreased effectiveness of diuretic medications, decreased urate excretion, hyperkalemia, analgesic nephropathy
- Central nervous system: Headache, dizziness, vertigo, confusion, depression, lowering of seizure threshold, hyperventilation (salicylates)
- Hypersensitivity: Vasomotor rhinitis, asthma, urticaria, flushing, hypotension, shock.
- Hepatotoxicity [8][7][21]
For a complete list of adverse effects for a particular NSAID, please see the StatPearls article for the specific drug.
- Antidepressant medications:
- Amitriptyline: Altered mental status, arrhythmias, constipation, decreased libido, dizziness, drowsiness, dry mouth, headache, hyperhidrosis, increased risk of suicidal thoughts, micturition disorders (i.e., urinary retention), nausea, orthostatic hypotension, tremor, weight gain.
- Duloxetine: Nausea, headache, dry mouth, somnolence, dizziness, abdominal pain, constipation increased blood pressure, increased risk of suicidal thoughts.[7][9][28][9]
- Antiepileptic medications:
- Gabapentin: The most common side effects are dizziness, somnolence, ataxia, peripheral edema, and confusion. Among other, more serious adverse effects are anaphylaxis, suicidality, depression, fever, infection, steven-Johnson syndrome, angioedema, erythema multiforme, and rhabdomyolysis.[29][31]
- Pregabalin: Dizziness, somnolence, headache, peripheral edema, nausea, weight gain, disorientation, blurred vision, increased risk of suicidal thoughts.[7]
- Local anesthetics:
- Lidocaine: Application-site pain, pruritus, erythema, and skin irritation.[7]
Opioid agents
Opioids produce a variety of different systemic adverse effects, including:
- Dysphoria/euphoria
- Sedation,
- Constipation
- Nausea and vomiting
- Cough suppression
- Miosis
- Histamine release (urticaria, pruritus, hypotension, tachycardia)
- Endocrine systems suppression
- Cardiovascular disorders (i.e., bradycardia)
- Respiratory depression
- Skeletal muscle rigidity
- Tolerance (chronic application)
- Physical dependence (chronic application)
- Opioid-induced hyperalgesia and/or allodynia (chronic application) [17][12]
Contraindications
Nonopioid analgesic agents
- Acetaminophen (paracetamol): Hypersensitivity to acetaminophen or any of its excipients, severe hepatic impairment, or severe active hepatic disease. Currently, experts are discussing whether hepatic impairment should be considered a contraindication since it would produce lower levels of the toxic metabolite, N-acetyl-p-benzoquinone imine (NAPQI).[6][32] It is worth noting that pregnancy is not a contraindication for acetaminophen administration.
- Nonsteroidal anti-inflammatory drugs (NSAIDs): Hypersensitivity is the only major contraindication for NSAID use. However, multiple conditions demand avoidance, temporary suspension, or extremely cautious use, as follows:
- Age > 50 years and family history of gastrointestinal (GI) disease/bleeding.
- Previous GI problems associated with NSAID use (eg., gastritis)
- Peptic ulcer
- History of personal GI bleeding
- Uncontrolled hypertension
- Renal disease
- Irritable bowel syndrome
- Inflammatory bowel disease
- Coronary artery bypass surgery
- Gastric bypass surgery
- Pregnancy (third trimester)
- Stroke (excluding aspirin)
- Transient ischemic attack (excluding aspirin)
- Myocardial infarction (excluding aspirin)
- Congestive heart failure (excluding aspirin) [7][21]
- Antidepressant medications:
- Amitriptyline: Hypersensitivity, coadministration with or within 14 days of monoamine oxidase inhibitors (MAOIs), coadministration with cisapride, recent myocardial infarction, arrhythmias, acute heart failure, severe liver impairment.
- Duloxetine: Hypersensitivity, liver impairment, severe renal failure (e.g., CrCl <30 mL/minute) or end-stage renal disease (ESRD), coadministration with or within 14 days of MAOIs, concomitant use of linezolid, thioridazine, methylene blue, or potent CYP1A2 inhibitors, uncontrolled narrow-angle glaucoma. Precaution in patients with hypertension or cardiac disease.[7][9][22]
- Antiepileptic medications: The only established contraindication for gabapentin and pregabalin is hypersensitivity to the respective drug or any of its excipients. However, dose adjustment is necessary for patients with compromised renal function.[7]
- Local anesthetics: Lidocaine is contraindicated in patients with a previous history of sensitivity to any local anesthetic of the amide-type or any its excipients. Additionally, the application of lidocaine patches should only be to intact skin.[24]
Opioid agents
- Severe respiratory instability
- Acute mental instability or high suicidal risk
- QTc interval over 500 milliseconds (methadone)
- Family and/or personal history of substance abuse
- Intolerance, serious adverse effects or lack of efficacy to other structurally similar opioids
- Renal or hepatic impairment (depending on the specific drug metabolism and excretion)[17]
Monitoring
Nonopioid analgesic agents
- Acetaminophen (paracetamol): It is considered a safe medication if used appropriately. Since toxicity usually occurs at doses above 150 mg/kg, the therapeutic index is estimated to be around 10.[33]
- Nonsteroidal anti-inflammatory drugs (NSAIDs): There is no need for routine monitoring of NSAID acute administration in healthy patients. However, patients who use NSAIDs chronically (eg., rheumatoid arthritis) and patients considered at increased risk for NSAID toxicity (eg., liver or renal disease) should have an evaluation including CBC, renal, and hepatic function tests as a minimum. Other tests may be ordered depending on clinical suspicion and findings.[8]
- Antidepressant medications:
- Amitriptyline: Allow 6 to 8 weeks for an adequate trial. If patients achieve adequate pain relief but do not tolerate the adverse reactions, consider switching to another TCA such as imipramine or nortriptyline. Consider switching the dose to bedtime to treat concurrent insomnia or if the patient experiences daytime somnolence. Patients should be monitored periodically with the following parameters: heart rate, blood pressure, ECG (older adults or preexisting cardiac disease), blood glucose, weight and BMI, electrolyte panel (high-risk population). Additionally, patients should undergo evaluation for suicide ideations and mood lability.
- Duloxetine: Allow 6 to 8 weeks for an adequate trial. Patients should be monitored periodically with blood pressure measurements (especially in patients with hypertension), liver and renal function tests (as clinically indicated), blood glucose and HbA in diabetic patients, and serum sodium in populations at high risk for complications. Additionally, clinicians should evaluate patients for suicide ideations.[7][9][28]
- Antiepileptic medications: Healthcare personnel should evaluate baseline creatinine levels before and during the treatment for patients under treatment for either gabapentin or pregabalin. Additionally, patients should do followed up for periodical screenings of depression, behavioral changes, and suicidality.[31][34]
- Local anesthetics: Since lidocaine has a narrow therapeutic index, patients with severe hepatic impairment, under prolonged infusions, or with broken or inflamed skin should be monitored for increased plasma levels. Additionally, there are documented cases of methemoglobinemia with a related use of local anesthetics, although thee are extremely rare related to patches administration. Signs of methemoglobinemia include cyanotic skin discoloration and/or abnormal coloration of the blood.[24]
Opioid agents
Clinicians should evaluate their patients on a periodical basis. Follow-ups should focus on the level of pain control and the physical examination (vital signs, signs of misuse, abuse, or addiction; respiratory and mental status; signs or symptoms of hypogonadism or hypoadrenalism).[17]
Toxicity
Nonopioid analgesic agents
- Acetaminophen (paracetamol): Toxicity develops at 7.5 g/day to 10 g/day or 140 mg/kg, it is rare at doses of less than 150 mg/kg for an adult or 200 mg/kg for a child. If taken in large amounts, this medication may cause severe liver damage leading to liver transplant or even death. Although acetaminophen poisoning is observed more frequently in children, adults usually present with a more serious and fatal presentation.[5][26][35][36] In cases of acute overdose, acetaminophen serum levels must be drawn between 4 to 24 hours since the estimated time of ingestion to determine treatment modality. The tool used to compare toxic levels is known as the Rumack-Matthew Nomogram. An acetaminophen level greater than 140 mcg/mL at 4 hours from ingestion requires appropriate treatment with N-acetyl-cysteine (NAC). Activated charcoal avidly binds acetaminophen and may be administered in cases that present within the first hour. However, it should not be administered in patients with altered consciousness or patients with increased risk of airway obstruction.[26][37][38][39]
- Nonsteroidal anti-inflammatory drugs (NSAIDs): Most cases of acute NSAID overdose are asymptomatic or develop insignificant self-limiting gastrointestinal symptoms. However, serious complications may occur and include confusion, headache, nystagmus, drowsiness, blurred vision, diplopia, tinnitus, convulsions, metabolic acidosis, acute renal or liver failure, GI bleeding, and coma.[8][40]
- Antidepressant medications:
- Amitriptyline: Severe signs and symptoms of toxicity include confusion, transient visual hallucinations, mydriasis, agitation, dysrhythmias, severe hypotension, convulsions, hyperreflexia, stupor, drowsiness, muscle rigidity, vomiting, hypothermia, hyperpyrexia, coma, and death. Alteration in the ECG, particularly in QRS axis or width, are clinically significant indicators of TCA toxicity. Other abnormalities include prolonged PR interval, ST-T wave changes, ventricular tachycardia, and fibrillation. The absence of these findings does not exclude TCA poisoning.[7][9][28]
- Duloxetine: Signs and symptoms of toxicity include serotonin syndrome, somnolence, syncope, tachycardia, seizures, autonomic instability, diarrhea, vomiting, and coma. There is no antidote available for duloxetine overdose. Nonetheless, cyproheptadine and cooling measures merit consideration in cases of serotonin syndrome.[41]
- Antiepileptic medications:
- Gabapentin: Gabapentin does not carry the risk of overdose or addiction as other pain management medications (eg., opioids). However, gabapentin may increase a euphoric state caused by opioids.[31]
- Pregabalin: Limited information is available regarding overdose. The highest documented accidental overdose occurred during clinical research and was 8000 mg. As in the case of gabapentin, pregabalin has no specific antidote.[34]
- Local anesthetics: Lidocaine toxicity usually presents at lidocaine blood concentrations above 5 mcg/mL. Symptoms progress from slurred speech, tinnitus, paresthesias, and dizziness to loss of consciousness, seizures, cardiac arrhythmias, and cardiorespiratory arrest. Management is supportive (oxygen, IV fluids, and inotropes), and, in cases of refractory cardiovascular collapse, intravenous lipid emulsion is indicated.[24]
Opioid agents
Opioid overdose may result in death due to severe respiratory depression. Physicians should suspect opioid toxicity in any patient presenting with altered mental status, bradypnea, and constricted pupils. Naloxone is indicated for patients with respiratory depression; it may be given intravenously, intramuscularly, or intranasally. Since naloxone is only active for 30 to 60 minutes, it must be administered through intravenous infusion in cases of a long-acting opioid overdose.[17][42]
Enhancing Healthcare Team Outcomes
Pain management requires a multidisciplinary healthcare team to address accurately and individually pain control for patients. Because adverse effects tend to occur at a much higher rate in patients with specific comorbidities, follow-ups must include a complete history and physical exam to alert for side effects or signs of addiction/misuse.
Patient education is crucial; providers need to pay close attention to the patient's symptoms and complaints to provide the best healthcare possible and avoid any adverse systemic effect. Several methods exist to detect any type of drug misuse appropriately. They include the following: state prescription drug monitoring programs, assessment surveys, adherence check-lists, motivational counseling, urine screening, and dosage forms verification. Through collaborative interprofessional teamwork, pain management can confer maximum benefit with minimal downside.
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)