West Virginia Opioid Prescribing For Chronic Pain While Avoiding Drug Diversion
- Article Author:
- Alexander Dydyk
- Article Author:
- Daniel Sizemore
- Article Author:
- Lindsay Trachsel
- Article Author:
- Till Conermann
- Article Editor:
- Burdett Porter
- Updated:
- 11/6/2020 6:54:27 AM
- For CME on this topic:
- West Virginia Opioid Prescribing For Chronic Pain While Avoiding Drug Diversion CME
- PubMed Link:
- West Virginia Opioid Prescribing For Chronic Pain While Avoiding Drug Diversion
Introduction
Chronic pain and opioid use and abuse is a significant problem in the United States.[1] Over one-quarter of United States citizens suffer from chronic pain.[2] It is among the most common complaints seen in an outpatient clinic and in the emergency department. The failure to manage chronic pain, as well as the possible complication of opioid dependence related to treatment, can result in significant morbidity and mortality. One in five patient complaints in an outpatient clinic is related to pain, with over half of all patients seeing their primary care provider for one pain complaint or another. It is paramount that providers have a firm grasp on the management of patients with chronic pain. As a country, the United States spends well over 100 billion dollars a year on healthcare costs related to pain management and opioid dependence.[3] Pain-related expenses exceed those for the costs of cancer, diabetes, and heart disease combined.[4] How a patient's chronic pain gets managed can have profound and long-lasting effects on a patient's quality of life.
The International Association for the Study of Pain defines chronic pain as any pain lasting longer than three months.[5] There are multiple sources of chronic pain. Combination therapy for pain includes both pharmacological therapies and nonpharmacological treatment options. There is a more significant reduction in pain with combination therapy compared to a single treatment alone. Escalation of pharmacological therapy is in a stepwise approach. Comorbid depression and anxiety are widespread in patients with chronic pain. Patients with chronic pain are also at increased risk for suicide. Chronic pain can impact every facet of a patient's life. Thus learning to diagnose and appropriately manage patients experiencing chronic pain is critical.[6]
Unfortunately, studies have revealed an inherent lack of education regarding pain management in most medical schools and training programs. The Association of American Medical Colleges recognized the problem and has encouraged schools to commit to opioid-related education and training by incorporating the Centers for Disease Control and Prevention guidelines for prescribing opioids for chronic pain into the medical school curriculum.
Appropriate opioid prescribing includes prescribing sufficient opioid medication through regular assessment, treatment planning, and monitoring to provide effective pain control while avoiding addiction, abuse, overdose, diversion, and misuse. To be successful, clinicians must understand appropriate opioid prescribing, assessment, the potential for abuse and addiction, and potential psychological problems. Inappropriate opioid prescribing typically involves not prescribing, under prescribing, overprescribing, or continuing to prescribe opioids when they are no longer effective.
The American Society of Addiction Medicine describes addiction as a treatable chronic disease that involves environmental pressures, genetics, an individual's life experiences, and interactions among brain circuits. Individuals that become addicted to opioids or other medications often engage in behaviors that become compulsive and result in dangerous consequences. The American Society of Addiction Medicines notes that while the following should not be used as diagnostic criteria due to variability among addicted individuals, they identify five characteristics of addiction:
- Craving for drug or positive reward
- Dysfunctional emotional response
- Failure to recognize significant problems affecting behavior and relationships
- Inability to consistently abstain
- Impairment in control of behavior
Unfortunately, most health providers' understanding regarding addiction is often confusing, inaccurate, and inconsistent due to the broad range of perspectives of those dealing with patients suffering from addiction. While a knowledge gap is present among healthcare providers, it is equally prevalent in politicians writing laws and law enforcement attempting to enforce the laws they write. Payers are responsible for the expenses associated with the evaluation and treatment of addiction. Persistent lack of education and the use of obsolete terminology continue to contribute to a societal lack of understanding for effectively dealing with the challenges of addiction.
In the past, the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders defined "addiction," "substance abuse," and "substance dependence" separately. The result was provider confusion contributed to the under-treatment of pain. Over time, the manual has eliminated these terms and now uses "substance use disorder," ranging from mild to severe.
Unfortunately, there are numerous challenges in pain management, such as both underprescribing and overprescribing opioids. The concerns are particularly prominent in patients with chronic pain and have resulted in patients suffering from inadequately treated pain while at the same time there has been a development of concomitant opioid abuse, addiction, diversion, and overdose. As a result, providers are often negatively influenced and fail to deliver appropriate, effective, and safe opioids to patients with chronic pain. Providers have, in the past, been poorly trained and ill-informed in their opioid prescribing. To make the challenges even worse, chronic pain patients often develop opioid tolerance, significant psychological, behavioral, and emotional problems, including anxiety and depression related to under or overprescribing opioids.
Clinicians that prescribe opioids face challenges that involve medical negligence in either failure to provide adequate pain control or risk of licensure or even criminal charges if it is perceived they are involved in drug diversion or misuse. All providers that prescribe opioids need additional education and training to provide the best patient outcomes and avoid the social and legal entanglements associated with over and under prescribing opioids.
Provider Opioid Knowledge Deficit
There are substantial knowledge gaps around appropriate and inappropriate opioid prescribing, including deficits in understanding current research, legislation, and appropriate prescribing practices. Providers often have knowledge deficits that include:
- Understanding of addiction
- At-risk opioid addiction populations
- Prescription vs. non-prescription opioid addiction
- The belief that addiction and dependence on opioids is synonymous
- The belief that opioid addiction is a psychologic problem instead related to a chronic painful disease
With a long history of misunderstanding, poor society, provider education, and inconsistent laws, the prescription of opioids has resulted in significant societal challenges that will only resolve with significant education and training.
Etiology
Most patients who suffer from chronic pain complain of more than one type of pain.[7] For example, a patient with chronic back pain may also have fibromyalgia. A significant percentage of patients suffer from a major depressive and generalized anxiety disorder. Over 67% of patients with chronic pain suffer from a comorbid psychiatric disorder.[8]
There are multiple categories and etiologies of types of pain, which include neuropathic, nociceptive, musculoskeletal, inflammatory, psychogenic, and mechanical.[6][9]
Neuropathic Pain
- Peripheral neuropathic pain, as in the case of post-herpetic neuralgia or diabetic neuropathy
- Central neuropathic pain - cerebral vascular accident sequella
Nociceptive Pain
- Pain due to actual tissue injuries such as burns, bruises, sprains, or fractures.
Musculoskeletal Pain
- Back pain
- Myofascial pain
Inflammatory Pain
- Autoimmune disorders (rheumatoid arthritis)
- Infection
Psychogenic Pain
- Pain caused by psychologic factors such as headaches or abdominal pain caused by emotional, psychological, or behavioral factors
Mechanical Pain
- Expanding malignancy
Epidemiology
Chronic Pain
There are over 100 million people in the United States who would meet the criteria for chronic pain syndrome.[2] Over 50 million Americans suffer from debilitating chronic pain, and over 20 million indicate that pain interferes with their daily lives. Chronic regional pain is reported in 11.1 percent of chronic pain patients, while chronic back pain accounts for 10.1 percent, leg and foot pain 7.1 percent, arm and hand pain 4.1 percent, and headache 3.5 percent. There are reports of widespread pain in 3.6% of patients with chronic pain.[8] Elderly patients have been shown to receive up to 25% fewer pain medications than the average population.[10]
Chronic pain is estimated to cost over $600 billion annually in lost productivity and medical treatment. Lifetime chronic pain is common, with over 50% of adults being affected at some point in their lives. Over 40% of chronic pain patients indicated their pain is not controlled, and over 10% suffer long-term disabling chronic pain.
Research has shown the lifetime prevalence for chronic pain patients attempting suicide between 5% and 14%; suicidal ideation was approximately 20%.[11] Of the chronic patient patients who commit suicide, 53.6% died of firearm-related injuries, while 16.2% by opioid overdose.[12]
The incidence of chronic pain is increasing due to the prevalence of obesity-related pain conditions, increased survival of trauma and surgical patients, an aging population, and heightened public awareness of pain as a treatable condition.
Opioids
Opioids are the most used therapeutic agent for chronic pain and are derived synthetically from generally unrelated compounds. Opiates are derived from the liquid of the opium poppy either by direct refinement or by relatively minor chemical modifications. Both opioids and opiates act on three major classes of opioid receptors: mu, kappa, delta, and several minor classes of opioid receptors like nociceptin and zeta. Simplifying significantly, the mu receptors are thought to provide analgesia, respiratory suppression, bradycardia, physical dependence, gastrointestinal dysmotility, and euphoria. The kappa agonism can yield hallucinations, miosis, and dysphoria. The delta receptor likely has pain control and mood modulation effects, but some have suggested that mu agonism is necessary for the delta receptor to function strongly for analgesia.[13][14] The nociceptin receptor modulates brain dopamine levels and has clinical effects like analgesia and anxiolysis. The zeta receptor, also known as the opioid growth factor receptor, can modulate certain types of cell proliferation, such as skin growths, and are not thought to have many functions in modulating pain or emotion.[15][16]
In the past, providers in the United States rarely prescribed opioids for any condition except chronic cancer pain. This approach began to change in the 1990s. Dr. James Campbell addressed the American Pain Society (now bankrupt) in 1995 and urged healthcare providers to treat pain as the fifth vital sign.[17] The prescription of opioids for treating all chronic pain conditions has grown to the point that opioid sales have reached over 7 billion dollars. The United States currently consumes more than 80% of all opioids produced worldwide. With increased use, concomitant problems have developed, and the number of individuals abusing opioid analgesics has increased dramatically.[18][19]
West Virginia has been substantially affected by the diversion of prescription drugs, particularly hydrocodone and oxycodone. Hydrocodone and oxycodone are the most abused prescription drugs in West Virginia. With the education of providers on inappropriate prescribing of opioids and a slowing of diversion, individuals dependent on opioids have unfortunately shifted to the use of heroin and fentanyl. The death rate of overdose death due to prescription drugs has decreased while overdose deaths to non-prescription opioids have climbed rapidly. In fact, West Virginia has experienced the highest drug overdose rate in the country, with approximately 50 deaths per 100,000 population.[20]
Pathophysiology
The pathophysiology of acute and chronic pain is complex and not completely understood. Research is ongoing. In general, the following provides an overview of the current understanding of pain pathophysiology.[6][9]
Acute Pain
It is caused by the activation of peripheral pain receptors and specific A-delta and C-sensory nerve fibers (nociceptors). It is commonly a response to tissue injury.
Chronic Pain
Chronic pain is usually related to ongoing tissue injury is thought to be caused by persistent activation of these pain fibers. Chronic pain may also develop from ongoing damage to or dysfunction of the peripheral or central nervous system which causes neuropathic pain.
Nociceptive Pain
Nociceptive pain receptors may be somatic or visceral. Somatic pain receptors are located in the subcutaneous tissues, fascia, connective tissues, endosteum, periosteum, joint capsules, and skin. Stimulation results in burning, dull, or sharp pain.
Visceral pain receptors are located in the viscera and connective tissue. Visceral pain due is usually due to obstruction of a hollow organ. This pain is deep, cramping, poorly localized, and may be referred to a remote site. Visceral pain from injury of connective tissue or organ capsules may be more localized and sharp.
Psychologic Factors
Psychologic factors in the modulation of pain intensity are highly variable. Culture, emotions, and thoughts and emotions affect an individual's perception of pain. Patients who suffer from chronic pain may also have psychological stress, particularly anxiety and depression. As a result of some syndromes being characterized as psychiatric disorders such as somatic symptom disorders defined by self-reported pain, patients are often mischaracterized as having a psychiatric disorder and are deprived of appropriate care. Pain may interfere with cognitive attention, concentration, the content of thought, and memory.
Multifactorial Pain Syndromes
Many pain syndromes are multifactorial; for example, cancer and chronic low back pain syndromes have a nociceptive component but may also involve neuropathic pain as a result of nerve damage.
Pain Modulation and Transmission
Pain fibers enter the spinal cord at the posterior root ganglia and synapse in the posterior horn. Fibers then cross to the contralateral side and travel up the lateral columns reaching the thalamus and, finally, the cerebral cortex. Repetitive stimulation from prolonged pain can sensitize neurons in the posterior horn of the spinal cord so that a minimal peripheral stimulus results in pain.
Peripheral and nerves at other levels of the central nervous system are sensitized, resulting in long-term synaptic changes in cortical receptive fields that can result in exaggerated pain perception. This process of chronic afferent input causing increased sensitivity and lower pain thresholds, with the remodeling of the central nociceptive pathways and receptors termed central sensitization. The consequence may be:
- Allodynia (exaggerated response to a nonpainful stimulus)
- Hyperalgesia (excessive pain response to a normal pain stimulus)
Substances Released When Tissue is Injured
The inflammatory cascade can sensitize peripheral nociceptors. Substances released include vasoactive peptides such as calcitonin, gene-related protein, neurokinin A, substance P, and mediators such as bradykinin and epinephrine prostaglandin E2, and serotonin. The pain signal is modulated in both segmental and descending pathways by neurochemical mediators such as endorphins (enkephalin) and monoamines (norepinephrine, serotonin). These mediators are thought to increase, reduce, shorten sustain the perception and response to pain. They also mediate the benefits of CNS-active drugs such as antidepressants, antiseizure drugs, membrane stabilizers, and opioids that interact with neurochemicals and specific receptors that treat chronic pain.
Psychologic factors are important pain modulators. They affect how patients describe and react to pain; such as complaining, being irritable, grimacing, or being stoic. They may also generate neural output that modulates neurotransmission along pain pathways. The psychologic reaction to protracted pain interacts with central nervous system factors that may induce long-term changes in pain perception.
Pain Theories
Intensity Theory
The theory goes back to the Athenian philosopher Plato (c. 428 to 347 B.C.) who, in his work Timaeus, defined pain not as a unique experience, but as an 'emotion' that occurs when the stimulus is intense and lasting. Centuries later, we are aware that especially chronic pain represents a dynamic experience, profoundly changeable in a spatial-temporal manner. A series of experiments, conducted during the nineteenth century, sought to establish the scientific basis of the theory. Based on the tactile stimulation and impulses of other nature, such as electrical stimulations, these investigations provided important information concerning the threshold for tactile perceptions and on the role of the dorsal horn neurons in the transmission/processing of pain.[21]
Cartesian Dualistic Theory
The oldest explanation for why pain manifested in specific populations was rooted in religious beliefs. Throughout history, religious ideologies have had a substantial influence on people’s thoughts and actions. As a result, the majority of people believed that pain was the consequence of committing immoral acts. There was also a belief that the suffering they endured was the individual’s way to repent for these sins.[9] Although this belief remained popular up until the nineteenth century, this was not due to the lack of other available theories. One of the first alternative scientific pain theories was bravely introduced in 1644 by the French philosopher Renee Descartes (1596-1650). This theory has the name in current literature as the Cartesian dualism theory of pain.[10]
The dualism theory of pain hypothesized that pain was a mutually exclusive phenomenon. Pain could be a result of physical injury or psychological injury. However, the two types of injury did not influence each other. At no point were they to combine and create a synergistic effect on pain, hence making pain a mutually exclusive entity.[11] In an attempt to placate the church, Descartes also included in his theory the idea that pain has a connection to the soul. He claimed that his research uncovered that the soul of pain was in the pineal gland, consequentially designating the brain as the moderator of painful sensations.[12]
The dualistic approach to pain theory fails to account for many factors that are known to contribute to pain today. Furthermore, it lacks explanation as to why no two chronic pain patients have the same experience with pain even if they had similar injuries. Despite these shortcomings, it still provided future researchers with a solid foundation to continue expanding the scientific understanding of the intricate phenomenon of pain.
Specificity Theory
Many scientists continued to do research long after Descartes proposed the dualistic theory of pain. However, it wasn’t until 1811 that another well-known pain theory came onto the scene. This theory, initially presented by Charles Bell (1774–1842), is referred to as the specificity theory. This theory is similar to Descartes' dualistic approach to pain in the way that it delineates different types of sensations to different pathways. In addition to the identification of specific pathways for different sensory inputs, Bell also postulated that the brain was not the homogenous object that Descartes believed it was, but instead a complex structure with various components.[13]
Scientists and philosophers alike spent the next century and a half further developing the specificity theory. One of the many contributors to this theory was Johannes Muller. In the mid-1800s, Muller published in Manual of Physiology that individual sensations resulted from specific energy experienced at certain receptors. Furthermore, Muller believed that there was an infinite number of receptors in the skin. This surplus of receptors accounted for the ability of an individual to discriminate between different sensations.[14] In 1894, Maximillian von Frey made another critical addition to the specificity theory that served to advance the concept: four separate somatosensory modalities found throughout the body. These sensations include cold, pain, heat, and touch.[15]
This concept correlates well with previous research done regarding this theory of pain, which served to reiterate the presence of distinct pathways for different sensations. Although this theory and the research surrounding it provided significant advancement to understanding pain, it still fails to account for factors other than those of physical nature that results in the sensation of pain. Much like the dualistic approach to pain, this theory also lacks an explanation for why sometimes pain persists long after the healing of the initial injury. This incomplete nature of the specificity theory regarding pain etiology necessitated additional theories and continued research.
Pattern Theory
Following the specificity theory, there were a handful of other philosophies introduced regarding the sensation of pain. Of these philosophies, the pattern theory of pain has the greatest coverage in the scientific literature. The American psychologist John Paul Nafe (1888-1970) presented this theory in 1929. The ideas contained with the pattern theory were directly opposite of the ideas suggested in the Specificity theory in regards to sensation. Nafe indicated that there are not separate receptors for each of the four sensory modalities. Instead, he suggested that each sensation relays a specific pattern or sequence of signals to the brain. The brain then takes this pattern and deciphers it. Depending on which pattern the brain reads, correlates with the sensation felt.[16] At the time of its introduction, the pattern theory gained significant popularity among many researchers. However, through further research and the discovery of unique receptors for each type of sensation, it can be stated with certainty that this theory is an inaccurate explanation for how we feel pain.
Gate Control Theory
In 1965, Patrick David Wall (1925–2001) and Ronald Melzack announced the first theory that viewed pain through a mind-body perspective. This theory became known as the gate control theory.[17] Melzack and Wall’s new theory partially supported both of the two previous theories of pain but also presented more knowledge to advance the understanding of pain further. The gate control theory of pain states that when a stimulus gets sent to the brain, it must first travel to three locations within the spinal cord. These include the cells within the substantia gelatinosa in the dorsal horn, the fibers in the dorsal column, and the transmission cells located in the dorsal horn.[18] The substantia gelatinosa of the spinal cord's dorsal horn serves to modulate the signals that get through, acting similar to a “gate” for information traveling to the brain.[19]
The sensation of pain that an individual feels is the result of the complex interaction among these three components of the spinal cord. Simply stated, when the “gate” closes, the brain does not receive the information that is coming from the periphery to the spinal cord. However, when the signal traveling to the spinal cord reaches a certain level of intensity, the “gate” opens. Once the gate is open, the signal can travel to the brain where it is processed, and the individual proceeds to feel pain. The information mentioned above accounts for the physical component of pain. Still, as stated earlier, the Gate Control Theory was one of the first to acknowledge that psychological factors contributed to pain as well. In their original study, Melzack and Wall suggested that in addition to the control provided by the substantia gelatinosa, there was an additional control mechanism located in cortical regions of the brain.[20]
In more recent times, researchers have postulated that these cortical control centers are responsible for the effects of cognitive and emotional factors on the pain experienced. Current research has also suggested that a negative state-of-mind serves to amplify the intensity of the signals sent to the brain as well.[21] For example, somebody who is depressed has a “gate” that is open more often, allowing more signals to get through, increasing the probability that an individual will experience pain from an otherwise normal stimulus. Also, there are reports that certain unhealthy lifestyle choices will also result in an “open gate,” which in turn leads to pain that is disproportionate to the stimulus.[22]
The gate control theory has proven to be one of the most significant contributions to the study of pain throughout history. The concepts that Melzack and Wall introduced to the study of pain are still utilized by researchers today. Even though this theory initiated the idea that pain wasn’t solely a result of physical injury but rather a complex experience, influenced by cognitive and emotional factors, additional research was necessary to comprehend the mechanisms and etiology of pain completely. This need precipitated the introduction of the following two philosophies regarding pain.
Neuromatrix Model
Almost thirty years after introducing the gate control theory of pain, Ronald Melzack introduced another model that contributed to the explanation of how and why people feel pain. Until the mid-1900s, most pain theories implied that this experience was exclusively due to an injury that had occurred somewhere in the body. The thinking was that if an individual suffered an injury, whether it be through trauma, infection, or disease, a signal would transmit to the brain, which would, in turn, result in the sensation of pain. Although Melzack had contributed to these previous theories, his exposure to amputees experiencing phantom limb pain in well-healed areas prompted his inquiry into this more accurate philosophy of pain. The theory he proposed is known as the neuromatrix model of pain. This philosophy suggests that it is the central nervous system that is responsible for eliciting painful sensations rather than the periphery.[23]
The neuromatrix model denotes that there are four components within the central nervous system responsible for creating pain. The four components are the “body-self neuromatrix, the cyclic processing, and synthesis of signals, the sentinel neural hub, and the activation of the neuromatrix.”[24] According to Melzack, the neuromatrix consists of multiple areas within the central nervous system that contribute to the signal, which allows for the feeling of pain. These areas include the spinal cord, brain stem and thalamus, limbic system, insular cortex, somatosensory cortex, motor cortex, and prefrontal cortex.[25] The signal that these areas of the central nervous system work together to create is responsible for allowing an individual to feel pain, and he referred to as the “neurosignature.” Furthermore, this theory states that input coming in from the periphery can initiate or influence the neurosignature, but these peripheral signals cannot create a neurosignature of their own.[26]
This idea that peripheral signals can alter the neurosignature is an important concept when considering the effect of nonphysical factors on an individual’s experience with pain. Melzack’s theory claimed that not only are there specific neurosignatures that elicit certain sensations but when there is an alteration in a certain signal, this allows for memory formation of these particular experiences. If the same circumstances occur again in the future, this memory allows for the same sensation to be felt.[27] In addition to the hypothesis that pain was a product of different patterns of signals from the central nervous system, the neuromatrix model continued to elaborate on the idea that was initially brought forward in the gate control theory, that pain can be affected not only by physical factors but by cognitive and emotional factors. Melzack suggested that hyperactivity of the stress response has a direct effect on pain. Hyperactivity of the stress response is when an individual exposed to increased levels of stress experiences a higher level of pain.[28]
Taking all of these claims into consideration, it is evident that pain is a complex issue that cannot be accounted for by physical factors alone. Even though the neuromatrix model further established the idea that cognitive and emotional factors influence pain in addition to physical factors, it still fails to account for social constructs of pain. Therefore, a new theory of pain must be utilized to appropriately explain the mechanism behind pain and why each individual’s experience with pain is unique.
Biopsychosocial Model
The biopsychosocial model provides the most comprehensive explanation behind the etiology of pain. This specific theory of pain hypothesizes that pain is the result of complex interactions between biological, psychological, and sociological factors. Any theory that fails to include all of these three pain constructs fails to provide an accurate explanation for why an individual is experiencing pain.[29] Although the term biopsychosocial was not introduced until 1954 by Roy Grinker (1900-1993), a neurologist and psychologist, many physicians had considered the utility of using such a model to approach the management of a patient’s pain long before this.[30]
One of the most prominent physicians who utilized this more comprehensive approach to pain was John Joseph Bonica (1917-1994). This Sicilian American anesthesiologist at Madigan Army Hospital established himself as the founding father of the pain medicine discipline. In the 1940s, Bonica cared for many patients who had returned home from World War II and were now experiencing debilitating pain due to injuries they had suffered in the war. He had recognized that the pain these wounded soldiers were experiencing was rather complex and not easily managed. This situation led him to propose that to adequately manage these patients, physicians needed to create interprofessional pain clinics comprising multiple disciplines.[31] At this moment in history, there was little support for the idea that pain was more than just the result of an injury, and Bonica was relatively unsuccessful in establishing these clinics. It wasn’t until 1977 that the biopsychosocial model was scientifically suggested as an explanation for the etiology of some medical conditions. George Engle claimed that to treat disease adequately; one must consider multidimensional concepts and manage the whole patient instead of focusing on a single issue.[32]
This methodology takes into account that the human body cannot be divided into separate categories when considering treatment options. Instead, it is beneficial to acknowledge the fact that illness and disease are the results of complex interactions between biological, psychological, and sociological factors, and they all affect an individual’s physical and mental well-being.[33] Although Bonica had technically been the first physician to comprehend the importance of using a biopsychosocial approach to pain, John D. Loeser, another anesthesiologist, has been credited as the first person to use this model in association with pain.[34]
Loeser suggested that four elements need to be taken into consideration when evaluating a patient with pain. These elements include nociception, pain, suffering, and pain behaviors. Nociception is the signal that is sent to the brain from the periphery to alert the body that there is some degree of injury or tissue damage. On the other hand, pain is the subjective experience that occurs after the brain has processed the nociceptive input. The last two components of pain that merit consideration is suffering and pain behaviors. The thinking is that suffering is an individual’s emotional response to the nociceptive signals and that pain behaviors are the actions that people carry out in response to the experience of pain. Both of these can be either conscious or subconscious.[35]
Loeser’s four pain elements account for the biological, psychological, and sociological factors that can create or influence an individual’s experience with pain. Failing to consider any one of these four elements when determining the cause or establishing a management plan could be a consideration as inadequate assessment or care. With a better understanding of what is causing a patient to experience pain, the doctor is provided with a more accurate foundation to begin formulating a treatment plan. Loeser’s findings prove that the Biopsychosocial Model of pain offers the most comprehensive philosophy and provides the framework that is needed to start appropriate therapy to manage patients with chronic pain adequately.
Histopathology
Chronic pain and opioids generally do not cause any specific histopathology in and of themselves. However, there is a diversity of histopathologic changes that can occur in the presence of improper/recreational parenteral administration of opioids. There may be chronic tissue damage present due to the original assault.[6]
Toxicokinetics
Opioids have an extensive diversity of durations and intensities of effect. For example, alfentanil has a half-life of around 1.5 hours; whereas, methadone has a half-life of between 8 to 60 hours. Opioid uptake and effect can also vary by administration route, some examples being fentanyl patches or long-acting oral formulations of oxycodone and morphine. Some, such as diphenoxylate and loperamide, have almost no effect other than suppression of bowel motility. Opioids such as methadone can significantly prolong the QT interval. Opioids can sometimes precipitate serotonin syndrome, especially when given to patients already taking various psychoactive medications (antidepressant medications like SSRI). There is an evolving body of knowledge that the intensity and quality of response to opioids can vary significantly between patients, which can be unrelated to tolerance; this is likely related to genetics, but this is not well characterized at this time.[22][19]
History and Physical
History
History should include the onset of pain, description, mechanism of injury if applicable, location, radiation of pain, quality, severity, factors contributing to relief or worsening of the pain, frequency of the pain, and any breakthrough pain. A verbal numeric rating scale (VNRS) or number scale for pain is a common measure to determine the severity of pain, numbered from 0 to 10. This tool is commonly used for pain intensity. Furthermore, associated symptoms should be assessed, such as muscle spasms or aches, temperature changes, restrictions to range of motion, morning stiffness, weakness, changes in muscle strength, changes in sensation, and hair, skin, or nail changes.
In addition to the patient's symptoms, the significance of the impact of the pain in day-to-day function should be discussed, and a review of daily living activities. It is important to understand how chronic pain affects the patient’s quality of life. Is pain impacting relationships or hobbies? Does the patient find themselves becoming depressed? Is the patient able to sleep throughout the night or exercise regularly? Can the patient go to work without limitations? Are activities of daily living affected, such as toileting, dressing, bathing, walking, or eating limited or restricted?
Older adults are a specific population that often identifies as suffering from chronic pain. The self-reporting of pain can be difficult in this population. Self-reporting of pain is essential for the identification and treatment of pain, while the inability to describe or communicate pain leads to undertreatment. Often elderly patients describe pain differently than the average population complicating diagnosis.[23][10] Instead of pain, an elderly patient may complain of soreness or discomfort.[24]
There are multiple acronyms used to obtain the history of a patient's pain. Some of the most commonly used abbreviations are "COLDERAS" and "OLDCARTS. These acronyms summarize the character, onset, location, duration exacerbating symptoms, relieving symptoms, radiation of pain, associated symptoms, and severity of illness. "PQRST" stands for provocation or palliation, quality, radiation or region, severity, and timing.[25]
A multidimensional assessment of a patient's pain and the severity of their pain can be completed. A Pain, Enjoyment, General Activity (PEG) tool can aid the multidimensional assessment of patients in pain.[26] The PEG score focuses on function and quality of life. A chronic pain patient who experiences daily 7/10 pain is treated with both pharmacological and nonpharmacological therapies. Following treatment, their pain is 5/10. A few points might not seem like a significant difference, but if their enjoyment and quality of life and function are improving, treatment may have had a profound impact on the patient's life. The PEG tool is scored 0 to 10 for each category. The higher the score, the worse the function and uncontrolled pain.
The Four-item Patient Health Questionnaire or PHQ-4 is a combination of the PHQ9 and GAD7 assessment tools used to evaluate depression and anxiety, respectively.[27] The PHQ-4 should be used as a screening tool for all cases of chronic pain. If the score of the PHQ-4 is more significant than five, then a full GAD-7, PHQ-9, and the Primary Care PTSD screening tools are recommended.[28]
The Defense and Veterans Pain Rating Scale (DVRPS) is a five-item tool with a 0 to 10 out pain scale, as well as an assessment of the impact of pain on sleep, mood, stress, and activity levels.[29]
In children's self-reporting, behavioral observation scales are used to assess pain.[30] Age-based rating scales of pain can be used. Visual analogs are also often implemented. Typically visual analogs are done with pictures of faces in various degrees of distress. By adolescence, children usually can rate their pain on a numerical scale, similar to adults.[31]
The Pediatric Pain Questionnaire and the Adolescent and Pediatric Pain Tool are used to assess the location of a patient's pain as well. The patient is asked to draw on the body map where they feel pain.[32] The ideal age for these tools is age 10 years.
Observational pain assessment tools are used in populations who cannot self-report. The facial expression, fussiness, distractibility, ability to be consoled, verbal responsiveness, and motor control are observational findings used in such an assessment tool. Observational pain assessment in infants or young children can use the (r-FLACC) tool.[30][33] The tool is an acronym for Revised Face, Legs, Activity, Cry, Consolability.[34][35] Multiple other validated tools can be used, the one that is better than another is the NAPI tool. However, multiple tools have been used and are validated.[36][37][38][33]
Nonverbal children with neurologic impairment (NI) is a challenging population to assess pain. Caregivers are often needed to help determine changes in the patient's behavior. Grimacing, moaning, increased muscle tone, crying, arching, atypical behavior such as aggressive behavior are a few symptoms to monitor in this population. Nonverbal children with NI include the Revised Face, Legs, Activity, Cry, Consolability (r-FLACC) scale, and the Individualized Numeric Rating Scale (INRS). The assessment adds specific behavior for atypical presentations.[34][37]
The Brief Pain Inventory (BPI) can be used to assess patients' beliefs on pain and the impact of pain on their lives.[39][40] Separately, the McGill Pain Questionnaire (SF-MPQ-2) includes a drawing for the location of pain on the human body, a questionnaire regarding previous pain medication use, and past experiences with pain.[41] Neuropathic pain is assessable using the Neuropathic Pain Scale to follow responses to therapy.
Physical
A detailed physical, including musculoskeletal, neurologic, and psychiatric exam, should be completed, as well as a focused examination of the area of pain.
Evaluation
Chronic Pain Assessment
Standard blood work and imaging are not indicated for chronic pain, but the clinician can order it when specific causes of pain are suspected. This can be on a case-by-case basis. In some cases, urine toxicology is ordered to monitor compliance and to exclude the use of nonprescription drugs.
Psychiatric disorders can amplify pain signaling making symptoms of pain worse.[42] Furthermore, comorbid psychiatric disorders, such as major depressive disorder, can significantly delay the diagnosis of pain disorders.[43] Major depressive disorder and generalized anxiety disorder are the most common comorbid conditions related to chronic pain. There are twice as many prescriptions for opioids prescribed each year to patients with underlying pain and a comorbid psychiatric disorder compared to patients without such comorbidity.[24] Intuitively, this makes sense. For example, a patient suffering from depression often complain of fatigue, sleep changes, loss of appetite, and decreased activity. These symptoms can make their pain worse over time. It is also crucial to realize patients with chronic pain are at an increased risk for suicide and suicidal ideation.[11][12]
Simultaneously screening for depression is recommended for patients with chronic pain. The Minnesota Multiphasic Personality Inventory-II (MMPI-2) or Beck's Depression Scale are the two most commonly used tools. The MMPI-2 has been used more frequently for patients with chronic pain.[44][45]
Addiction Risk Assessment [18][46][47]
The clinician should consider information from the history and physical, family members, the state prescription monitoring program, and screening tools to assess the risk of developing an untoward behavioral response to opioids. Patients can be stratified to three risk levels:
- Low-risk: standard monitoring, vigilance, and care
- Objective signs and symptoms, localizable physical pathology
- Confirmatory testing such as physical exam findings, CT, MRI, etc.
- No individual or family history of substance abuse
- At most, mild medical or psychologic comorbidity
- Age < 45
- High pain tolerance
- Active coping strategies
- Willingness to participate in multimodal therapy
- Attempting to function at normal levels
- Moderate-risk: additional level of monitoring and more frequent provider contact
- Significant pain
- Defined pathology with objective signs and symptoms
- Confirmatory testing such as physical exam findings, CT, MRI, etc.
- Moderate psychologic problems controlled by therapy
- Moderate comorbidities well controlled by medical therapy and are not affected by opioids
- Mild opioid tolerance but not hyperalgesia without addiction or physical dependence
- Individual or family history of substance abuse
- Pain involving more than three regions of the body
- Moderate levels of pain acceptance
- Active coping strategies
- Willing to participate in multimodal therapy
- Attempting to function at normal levels
- High-risk: intensive and structured monitoring, frequent follow-up contact, consultation with addiction psychiatrist, and limited monthly prescription of short-acting opioids
- Significant widespread pain
- No objective signs and symptoms
- Pain involves more than 3 body regions
- Divergent drug-related behavior
- Individual or family history of addiction, dependency, diversion, hyperalgesia, substance abuse, or tolerance
- Major psychologic problems
- Age >45
- HIV-related pain
- High levels of pain exacerbation
- Poor coping strategies
- Unwilling to participate in multimodal therapy
- Not functioning at a normal lifestyle
Prescribing Opioids
Before prescribing opioids, complete a detailed patient history that includes:
- Indication requested for pain relief
- Location, nature, and intensity of pain
- Prior pain treatments and response
- Comorbid conditions
- Potential physical and psychologic pain impact on function
- Family support, employment, and housing
- Leisure activities, mood, sleep, substance use, and work
- Emotional, physical, or sexual abuse
When considering opioids, weigh the risks of abuse, addiction, adverse drug reactions, overdose, and physical dependence. If there are any special concerns, such as a history of substance abuse, consult a psychiatrist or addiction specialist. If current substance abuse, withhold prescribing until the patient is involved in an addiction treatment and monitoring program.
Assessment Tools [47]
Screening tools assist in determining risk level, and degree of monitoring and structure required for a treatment plan; however, their validity is not yet supported in the literature. Some examples of opioid tools include:
Brief Intervention Tool
Brief Intervention Tool is a 26-item "yes-no" questionnaire used to identify signs of opioid addiction or abuse. The items assess for problems related to drug use-related functional impairment.
CAGE, CAGE-AID, and CAGE-Opioid
CAGE (Cut down, Annoyed, Guilty, and Eye-opener) Questionnaire consists of four questions designed to assess alcohol abuse. CAGE-AID and CAGE-OPIOID are revised versions to assess the likelihood of current substance abuse.[48]
Current Opioid Misuse Measure (COMM)
The Current Opioid Misuse Measure is a 17-item patient self-report assessment designed to identify abuse in chronic pain patients. It identifies aberrant behaviors associated with opioid misuse in patients already receiving long-term opioid therapy.
Diagnosis, Intractability, Risk, and Efficacy (DIRE) Tool
The Diagnosis, Intractability, Risk, and Efficacy is a clinician-rated questionnaire used to predict patient compliance with long-term opioid therapy. Patients scoring low are poor candidates for long-term opioids.
Mental Health Screening Tool
The Mental Health Screening Tool is a five-item screen that evaluates feelings of calmness, depression, happiness, peacefulness, and nervousness in the past month. A low is an indicator that the patient should be referred to a pain management specialist.
Opioid Risk Tool
The Opioid Risk Tool is a five-item assessment to evaluate for aberrant drug-related behavior. It categorizes the patient into low, medium, or high levels of risk for aberrant drug-related behaviors based on question responses concerning previous alcohol, drug abuse, psychologic disorders, and other risk factors.
Pain Assessment and Documentation Tool (PADT)
Guidelines by the CDC, the Federation of State Medical Boards, and Joint Commission stress documentation from both a quality and medicolegal perspective. The Pain Assessment and Documentation Tool (PADT) was designed to help the clinician document appropriate information.
Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R)
The Screener and Opioid Assessment for Patients with Pain-Revised (SOAPP-R) is a screen with questions addressing the history of alcohol or substance use, cravings, mood psychologic status, and stress. The SOAPP-R helps assess the risk level of aberrant drug-related behaviors and the monitoring level needed.
VIGIL
- Verification: Is this a responsible opioid user?
- Identification: Is the identity of this patient verifiable?
- Generalization: Do we agree on mutual responsibilities and expectations?
- Interpretation: Do I feel comfortable allowing this person to have controlled substances?
- Legalization: Am I acting legally and responsibly?
Urine Drug Tests (UDT)
Urine drug tests evaluate the use of the medication prescribed and detect unsanctioned drug use. The CDC recommends drug testing before starting opioid therapy and at least annually.
On study suggests monitoring frequency based on risk level.[49]
| Low-Risk Level | UDT every 1-2 years | State drug monitoring program - 2x per year |
| Medium-Risk Level | UDT every 6-12 months | State drug monitoring program - 3x per year |
| High-Risk Level | UDT every 3 months | State drug monitoring program - 4x per year |
Testing is usually done with class-specific immunoassay drug panels; however, this may be followed with gas chromatography/mass spectrometry for specific metabolite detection. The test should identify the specific drug. If urine test results suggest aberrant opioid use, discuss the issue in a positive, supportive approach, and document the discussion.
Treatment / Management
Healthcare professionals who treat patients with chronic pain should understand best practices in opioid prescribing, approaches to pain assessment, pain management modalities, and appropriate use of opioids for pain control. Pharmacologic and nonpharmacologic approaches should be evaluated. Patients with moderate-to-severe chronic pain who have been assessed and treated with non-opioid therapy without adequate pain relief are candidates for opioid therapy. Initial treatment should be a trial of therapy, not a definitive course of treatment. The CDC has issued updated guidance on the prescription of opioids for chronic pain. These guidelines address when to initiate or continue opioids for chronic pain; opioid selection, dosage, duration, follow-up, and discontinuation; and assessing risk and addressing opioid use harm.
Recommendations are to refer a patient to pain management in the case of debilitating pain, which is unresponsive to initial therapy. The pain may be located at multiple locations, requiring multimodal treatment or increases in dosages for adequate pain control or invasive procedures to control pain. Treatment of both pain and a comorbid psychiatric disorder leads to a more significant reduction of both pain and symptoms of the psychiatric disorder.[50] Pain may also worsen concurrent depression; thus, the treatment of pain has demonstrated to improve the responses to the treatments for depression.[51] There are multiple pharmacological, adjunct, nonpharmacological, and interventional treatments for chronic, severe, and persistent pain.
The list of pharmacological options for chronic pain is extensive. This list includes nonopioid analgesics such as nonsteroidal anti-inflammatories (NSAIDs), acetaminophen, and aspirin. Additionally, medications such as tramadol, opioids, antiepileptic drugs (gabapentin or pregabalin) can be useful. Furthermore, antidepressants such as tricyclic antidepressants and SNRI’s, topical analgesics, muscle relaxers, N-methyl-d-aspartate (NMDA) receptor antagonists, and alpha 2 adrenergic agonists are also possible pharmacological therapies.
Treatment response can differ between individuals, but treatment is typically done in a stepwise fashion to reduce the duration and dosage of opioid analgesics. However, there is no singular approach appropriate for the treatment of pain in all patients.[52]
Chronic musculoskeletal pain is nociceptive pain. The treatment of such pain is in a stepwise approach but includes a combination of nonopioid analgesics, opioids, and nonpharmacological therapies. First-line therapy would be acetaminophen or NSAIDs. Both are effective for osteoarthritis and chronic back pain.[53][54][55] However, NSAIDs are relatively contraindicated in patients with a history of heart disease or myocardial infarction, renal disease, or patients on anticoagulation or with a history of ulcers.[56][57] There is limited evidence of which NSAID to use over another. One nonsteroidal antiinflammatory pharmacological agent may have a limited effect on a patient's pain while another may provide adequate pain relief. The recommendations are to try different agents before moving on to opioid analgesics.[58] Failure to achieve appropriate pain relief with either acetaminophen or NSAIDs can lead to considering opioid analgesic treatment.
Opioids are considered a second-line option; however, they may be warranted for pain management for patients with severe persistent pain or neuropathic pain secondary to malignancy.[59] There have been conflicting results on the use of opioids in neuropathic pain. However, for both short term and intermediate use, opioids are often used to treat neuropathic pain.[60] Opioid therapy should only start with extreme caution for patients with chronic musculoskeletal pain.[61] Side effects of opioids are significant and frequent and may include opioid-induced hyperalgesia, constipation, dependence, and sedation. For chronic musculoskeletal pain, they are not superior to nonopioid analgesics.[62][63]
Administration of opioid analgesics should be reserved for when alternative pain medications have not provided adequate pain relief or contraindicated and when pain is impacting the patient's quality of life. The potential benefits outweigh the short and long-term effects of opioid therapy. The patient must make an informed choice before starting opioid treatment after discussing the risks, benefits, and alternatives to opioids.[62][64][65] Patients taking opioids at greater than 100 morphine milligram equivalents per day are at significantly increased risk of side effects. Side effects of opioids such as respiratory compromise will increase as the dosages increase. Patients with chronic pain could benefit from a therapy program designed to wean them from opioid analgesics to a safer dosage.[66][67] Long-acting opioids should only be used over short-acting opioids in the setting of disabling pain, causing severe impairment to quality of life.[68]
There is an estimated 78 percent risk of an adverse reaction to opioids such as constipation or nausea, while there is a 7.5 percent risk of developing a severe adverse reaction ranging from immunosuppression to respiratory depression.[69] Patients with chronic pain who meet the criteria for the diagnosis of opioid use disorder should receive the option of buprenorphine to treat their chronic pain. Buprenorphine is a considerably better alternative for patients with very high daily morphine equivalents who have failed to achieve adequate analgesia.
Different types of pain also warrant different treatments. For example, chronic musculoskeletal back pain would be treated differently from severe diabetic neuropathy. A combination of multiple pharmacological therapies is often necessary to treat neuropathic pain. Less than 50% of patients with neuropathic pain will achieve adequate pain relief with a single agent.[70] Adjunctive topical therapy, such as lidocaine or capsaicin cream, can be utilized as well.[71][72]
The initial treatment of neuropathic is often with gabapentin or pregabalin. These are calcium channel alpha 2-delta ligands. They are indicated for postherpetic neuralgia, diabetic neuropathy, and mixed neuropathy.[73] There is limited evidence in the use of other antiepileptic medications to treat chronic pain, where many of these, such as lamotrigine, have a more significant side effect profile. The exception is carbamazepine in the treatment of trigeminal neuralgia and other types of chronic neuropathic pain.[74][75]
Alternatively, antidepressants such as dual reuptake inhibitors of serotonin and norepinephrine (SNRI) or tricyclic antidepressants (TCA) can is an option. Antidepressants are beneficial in the treatment of neuropathic pain, central pain syndromes, and chronic musculoskeletal pain. For neuropathic pain, antidepressants have demonstrated a 50 percent reduction of pain. Fifty percent is a significant reduction, considering the average decrease in pain from various pain treatments is 30%.[76][77]
The serotonin-norepinephrine reuptake inhibitor (SNRI) duloxetine is a useful treatment for treating chronic pain, osteoarthritis, and the treatment of fibromyalgia.[78] Furthermore, the efficacy of duloxetine in the treatment of comorbid depression is comparable to other antidepressants.[79][76] Venlafaxine is an effective treatment for neuropathic pain, as well.[80] A TCA can also be utilized, such as nortriptyline. TCA medications may require six to eight weeks to achieve its desired effect.[59]
Adjunctive topical agents such as topical lidocaine are a useful treatment for neuropathic pain and allodynia as in postherpetic neuralgia.[81][82] Topical NSAIDs have been shown to improve acute musculoskeletal pain, such as a strain, but are less effective in chronic pain. Yet, topical NSAIDs are more effective than controls in the treatment of pain related to knee osteoarthritis.[83][84] Separately, topical capsaicin cream is an option for chronic neuropathic or musculoskeletal pain unresponsive to other treatments.[85] Botulinum toxin has also demonstrated effectiveness in the treatment of postherpetic neuralgia.[86] The use of cannabis is also an area of interest in pain research. There is some evidence that medical marijuana can be an effective treatment of neuropathic pain, while the evidence is currently limited in treating other types of chronic pain.[87]
The list of nonpharmacological therapies for chronic pain is extensive. Nonpharmacological options include heat and cold therapy, cognitive behavioral therapy, relaxation therapy, biofeedback, group counseling, ultrasound stimulation, acupuncture, aerobic exercise, chiropractic, physical therapy, osteopathic manipulative medicine, occupational therapy, and TENS units. Interventional techniques can also be utilized in the treatment of chronic pain. Spinal cord stimulation, epidural steroid injections, radiofrequency nerve ablations, botulinum toxin injections, nerve blocks, trigger point injections, and intrathecal pain pumps are some of the procedures and techniques commonly used to combat chronic pain. TENS units' efficacy has been variable, and the results of TENS units for chronic pain management are inconclusive.[88] Deep brain stimulation is for post-stroke and facial pain as well as severe, intractable pain where other treatments have failed.[89] There is limited evidence of interventional approaches to pain management. For refractory pain, implantable intrathecal delivery systems are an option for patients who have exhausted all other options.
Spinal cord stimulators are an option for patients with chronic pain who have failed other conservative approaches. Most commonly, spinal cord stimulators are placed following failed back surgery but can also be an option for other causes of chronic pain such as complex regional pain syndrome, painful peripheral vascular disease, intractable angina, painful diabetic neuropathy, and visceral abdominal and perineal pain.[90][91][92][93][94] Spinal cord stimulators have shown a 50% reduction of pain compared to continued medical therapy.[95]
Differential Diagnosis
Pain is a symptom, not a diagnosis. Developing a differential diagnosis for a patient's chronic pain is based on assessing the possible underlying etiologies of the patient's pain. It is essential to determine what underlying injury or disease processes are responsible for the patient's pain since this requires the identification of effective treatment. For instance, it is crucial to determine if a patient's neuropathic pain is peripheral or central. In another example, if a patient suffers from severe knee pain, it is essential to consider whether or not the knee pain is secondary to severe osteoarthritis since the patient may benefit from an injection or possibly from a knee replacement. In contrast, if the knee pain were instead related to a different condition such as rheumatoid arthritis, infection, gout, pseudogout, or meniscal injury, very different treatments would be necessary.
The differential diagnosis for generalized chronic pain would include patients who develop allodynia from chronic opioids as well as patients suffering from a major depressive disorder, as well as other psychiatric or sleep disorders, including insomnia. Furthermore, autoimmune diseases such as lupus or psoriatic arthritis, fibromyalgia as well as central pain syndromes, should be considered in states involving wide-spread, generalized chronic pain states. The four main categories of pain are neuropathic, musculoskeletal, mechanical, and inflammatory. Persistent and under-treated painful conditions can lead to chronic pain. Thus chronic pain is often a symptom of one or multiple diagnoses and can become its diagnosis as it becomes persistent and the body's neurochemistry changes. It is critical to treat acute and subacute pain before chronic pain develops.
Treatment Planning
Opioid therapy should begin as a trial for a pre-defined period, usually less than 30 days. Treatment goals should be established prior to the initiation of opioid therapy. These include the level of relief of pain, anxiety, depression, the return of function while avoiding unnecessary opioid use. The plan should include therapy selection, progress measures, and additional consultations, evaluations, referrals, and therapies. The provider should:
- Start at the lowest possible dose and then titrate to effect
- Start with short-acting opioid formulations
- Discuss the need for frequent risk/benefit assessments
- Be instructed in the signs and symptoms of respiratory depression
- Reassess risk/benefit with each dose increase
- Decisions to titrate dose to 90 mg or more morphine equivalent dose should be justified
- Be knowledgeable of federal and state opioid prescribing regulations
- Be knowledgeable of patient monitoring, equianalgesic dosing, and cross-tolerance with opioid conversion
- Augment treatment with nonopioid or, if necessary immediate-release opioids over long-acting opioids
- Taper opioid dose whenever possible
Consent and Treatment[18]
The opioid prescription should include documented informed consent and a treatment agreement addressing:
- Drug interactions
- Physical dependence
- Side effects
- Tolerance
- Physical dependence
- Driving and motor skill impairment
- Limited evidence of long-term benefit
- Addiction, dependence, misuse
- Risk/benefit profile of the drug prescribed
- Signs/symptoms of overdose
Prescribing policies should be clearly described, including policies regarding the number and frequency of refills and procedures for lost or stolen medications.
Patient and Physician treatment Agreement
- The patient should agree to use medications safely, avoid "doctor shopping," and to consent to urine drug testing
- The prescriber should agree to address problems, followup visits, and scheduled refills
Reasons for opioid therapy change or discontinuation should be listed. Agreements can also include follow-up visits, monitoring, and safe storage and disposal of unused drugs. If the patient does not speak English, an interpreter should be used.
Discontinuing Opioid Therapy
Discontinuing opioid therapy should be based on a physician-patient discussion. Opioids should be discontinued when the pain has resolved, side effects develop, analgesia is inadequate, or quality of life is not improved, deteriorating function, or there is evidence of aberrant medication use. Opioids should be tapered slowly, and withdrawal should be managed by an addiction specialist.
Toxicity and Side Effect Management
The American Society of Interventional Pain Physicians guidelines recommends monitoring for opioid adherence, abuse, and noncompliance by urine drug tests and monitoring programs.
The treatment plan for opioid use in chronic pain should include frequent assessment of pain level, origin, and function. If there is a change of dosage or agent, the frequency of patient visits should be increased. Chronic response to opioids should be monitored by evaluating the 5 A's.
- Affect
- Aberrant drug-related behaviors
- Activities of daily living
- Adverse or side effects
- Analgesia
Signs and symptoms, if present, that suggests treatment goals are not being achieved include:
- Decreased appetite
- Excessive sleeping or day/night reversal
- Impaired function
- Inability to concentrate
- Mood volatility
- Lack of involvement with others
- Lack of re-engaging in life
- Lack of hygiene
The decision to change, continue, or terminate opioid therapy is based on achieving treatment objectives without adverse effects. Physicians, wherever possible, should work with pharmacists.
Acute Overdose Management[19]
Accidental or deliberate overdose is always a risk factor in patients taking opioids. The patient and family should be instructed in the signs and symptoms of an overdose and basic emergency management until paramedics' arrival.
The immediate response to overdose management is to secure the airway and breathing; however, survival is heavily dependent upon the rapid administration of an opioid antagonist. Many states, including West Virginia, allow naloxone distribution to the public. Licensed healthcare providers may prescribe opioid antagonists for at-risk individuals, relatives, or caregivers. Emergency medical service personnel, peace officers, and firefighters also have the drug available.
While opioid antagonists such as naloxone, naltrexone, and nalmefene are available, acute overdoses are usually treated with naloxone as it quickly reverses opioid-related respiratory depression. Naloxone competes with opioids at receptor sites in the brain stem, reversing desensitization to carbon dioxide and preventing respiratory failure.
The naloxone dose is 0.4 to 2 mg administered intravenously, intramuscularly, or subcutaneously. The dose may be repeated every 2 to 3 minutes. Naloxone is available in pre-filled auto-injection devices. Advanced Cardiac Life Support protocols should be continued while naloxone is being administered.
In West Virginia, pharmacists and pharmacy interns may dispense naloxone without a provider prescription. The pharmacist or intern is required to screen the potential recipient by asking if they have a known hypersensitivity to naloxone; provide appropriate counseling and information on the product including dosing, effectiveness, adverse effects, storage, shelf-life, safety, and contact information (1-844-HELP-4-WV) for access to substance abuse treatment and recovery services if the recipient indicates interest in such services. Patients receiving opioid treatment for chronic pain may benefit from education and naloxone being available at home to treat an overdose.[96]
Prognosis
Current chronic pain treatments can result in an estimated 30% decrease in a patient's pain scores.[52] A thirty percent reduction in a patient's pain can have significant improvements to patients' function and quality of life.[97] However, the long-term prognosis for patients with chronic pain demonstrates reduced function and quality of life. Improved outcomes are possible in patients with chronic pain improves with the treatment of comorbid psychiatric illness. Chronic pain increases patient morbidity and mortality, as well as increases rates of chronic disease and obesity. Patients with chronic pain are also at a significantly increased risk for suicide compared to the regular population.
Spinal cord stimulation results in inadequate pain relief in about 50% of patients. Tolerance can also occur in up to 20 to 40 percent of patients. The effectiveness of the spinal cord stimulation decreases over time.[98] Similarly, patients who develop chronic pain and are dependent on opioids often build tolerance over time. As the amount of morphine milligram equivalents increases, the patient's morbidity and mortality also increase.
Ultimately, prevention is critical in the treatment of chronic pain. If acute and subacute pain receives appropriate treatment, and chronic pain can be avoided, the patient will have limited impacts on their quality of life.
Complications
Chronic pain leads to significantly decreased quality of life, reduced productivity, lost wages, worsening of chronic disease, and psychiatric disorders such as depression, anxiety, and substance abuse disorders. Patients with chronic pain are also at a significantly increased risk for suicide and suicidal ideation.
Many medications often used to treat chronic pain have potential risks and side effects and possible complications associated with their use.
Acetaminophen is a standard pharmacological therapy for patients with chronic pain. It is taken either as a single agent or in combination with an opioid. The hepatotoxicity occurs with acetaminophen when exceeding four grams per day.[99] It is the most common cause of acute liver failure in the United States.[100] Furthermore, hepatotoxicity can occur at therapeutic doses for patients with chronic liver disease.[101]
Frequently used adjunct medications such as gabapentin or pregabalin can cause sedation, swelling, mood changes, confusion, and respiratory depression in older patients who require additional analgesics.[102] These agents require caution in elderly patients with painful diabetic neuropathy. Also, gabapentin or pregabalin, in combination with opioid analgesics, has been shown to increase the rate of patient mortality.[103]
Duloxetine can cause mood changes, headaches, nausea as well as other possible side effects, and should be avoided in patients with a history of kidney or liver disease.
Feared complications of opioid therapy include addiction as well as overdose resulting in respiratory compromise. However, opioid-induced hyperalgesia is also a significant concern. Patients become more sensitive to painful stimuli while on chronic opioids.[104] The long-term risks and side effects of opioids include constipation, tolerance, dependence, nausea, dyspepsia, arrhythmia (methadone treatment QT prolongation), and opioid-induced endocrine dysfunction, which can result in amenorrhea, impotence, gynecomastia, and decreased energy and libido. Also, there appears to be a dose-dependent risk of opioid overdose with increasing daily milligram morphine equivalents.
Complication rates for spinal could stimulators are high, ranging from five up to 40%.[105][106] Most commonly, lead migration occurs, causing inadequate pain relief, often requiring revision and anchoring.[107][108] Lead movement often occurs in the cervical region of the spinal cord, given an increased range of motion of the cervical vertebra.[109][110] Spinal cord stimulator lead fracture can occur in up to 9% of placements [111][112]. Seromas are also very common and may require surgical incision and drainage.[113][105] The risk of infection following a spinal cord stimulator placement is between 2.5 and 12 percent.[114][115] Lastly, direct spinal cord trauma could occur. The most significant infectious complication would be a spinal cord abscess. A dural puncture can cause a post-dural headache in up to 70% of patients.[116][117][113] The most significant adverse to spinal cord stimulator placement would be a spinal epidural hematoma. This emergency would require immediate neurosurgical decompression of the hematoma. The incidence of a spinal epidural hematoma is 0.71%.[118]
Drug Diversion and Drug Seeking[46]
Unfortunately, due to addiction or financial gain, some individuals seek prescribed opioids for illicit purposes. Prescription opioids may be obtained from a friend or relative, purchased from a black market drug dealer, obtained by doctor shopping and acquiring drugs from multiple prescribers, and theft from clinics, hospitals, or pharmacies.
- Aggressive demands for more opioids
- Asking for opioids by name
- Behaviors suggesting opioid use disorder
- Forged prescriptions
- Increased alcohol use
- Increasing medication dose without provider permission
- Injecting oral medications
- Obtaining medications from nonmedical sources
- Obtaining opioids from multiple providers
- Prescription loss or theft
- Reluctance to decrease opioid dosing
- Resisting medication change
- Requesting early refills
- Selling prescriptions
- Sharing or borrowing similar medications
- Stockpiling medications
- Unsanctioned dose escalation
- Using illegal drugs
- Using pain medications to treat other symptoms
Drug Diversion Interventions
Prescribers and dispensers can take several precautions to avoid drug diversion. Some approaches include:
- Communication among providers and pharmacies to avoid "doctor shopping"
- Educate patients on the dangers of sharing opioids
- Encourage patients to keep opioid medications in a private place
- Encourage patients to refrain from public disclosure of opioid use
- Report patient prescribing to state central database if available
If a patient is suspected of drug-seeking or diversion, consider the following actions:
Inquire about prescription and illicit drug use
- Obtain a urine drug screen
- Perform a thorough examination
- Perform pill counting
- Prescribe smaller quantities of the opioid
If a patient is abusing prescribed opioids, this is a violation of the treatment agreement. The provider may then chose to discharge the patient from their practice. If the relationship is terminated, the provider must do it legally. The provider should avoid patient abandonment, ending a relationship with a patient without consideration of continuity of care, and without providing notice to the patient. To avoid abandonment charges, the patient must be given enough advanced warning to allow them to secure another physician and facilitate the transfer of care
Patients with a substance abuse problem or addiction should be referred to a pain specialist. Theft or loss of controlled substances should be reported to the Drug Enforcement Administration. If drug diversion has occurred, the activity should be documented and reported to law enforcement.
Consultations
Seek consultation or patient referral when input from a pain specialist, addiction specialist, or psychiatrist is needed, particularly for long-term chronic pain management. Clinicians who prescribe opioids should be aware of opioid addiction treatment options.
Deterrence and Patient Education
Involvement of Patient and Family
The patient and family can assist in making informed decision-making regarding continuing or discontinuing opioid therapy. Family members are often aware of when a patient is depressed and less functional. Questions to ask the family include:
- Is the patient's day focused on taking opioid pain medication?
- What is the frequency of pain medication?
- Does the patient have any other alcohol or drug problems?
- Does the patient avoid activity?
- Is the patient depressed?
- Is the patient able to function?
What To Teach A Patient Taking Opioids
- Avoid driving or operating power equipment
- Avoid stoping opioids suddenly
- Avoid taking other drugs that depress the respiratory system as alcohol, sedatives, and anxiolytics
- Contact prescribing if pain medication is not adequate for relief
- Destroy opioids based on product-specific disposal information (usually flushing down the toilet or mixing with cat litter or coffee grounds)
- Do not chew tables
- Do not share opioids with friends or family
- Follow prescribed dosing regimen
- Provide product-specific information
- Take opioids only as prescribed
Pearls and Other Issues
Maintain Accurate Medical Records Regarding Opiate Prescriptions
All clinicians should maintain accurate, complete, and current medical records, including:
- Records of prescriptions for controlled substances
- Record instructions provided
- Detailed history, physical, monitoring, and reasons prescribed
Federal and State Laws[119]
Several regulations and programs at the federal and state level to reduce prescription opioid abuse, diversion, and overdose. These laws require:
- Immunity from prosecution for individuals seeking assistance during an overdose
- Pain clinic oversight
- Patient identification prior to dispencing
- Physical examination prior to prescribing opioids
- Prescription limits
- Prohibition from obtaining controlled substance prescriptions
- Tamper-resistant prescriptions
Federal Laws
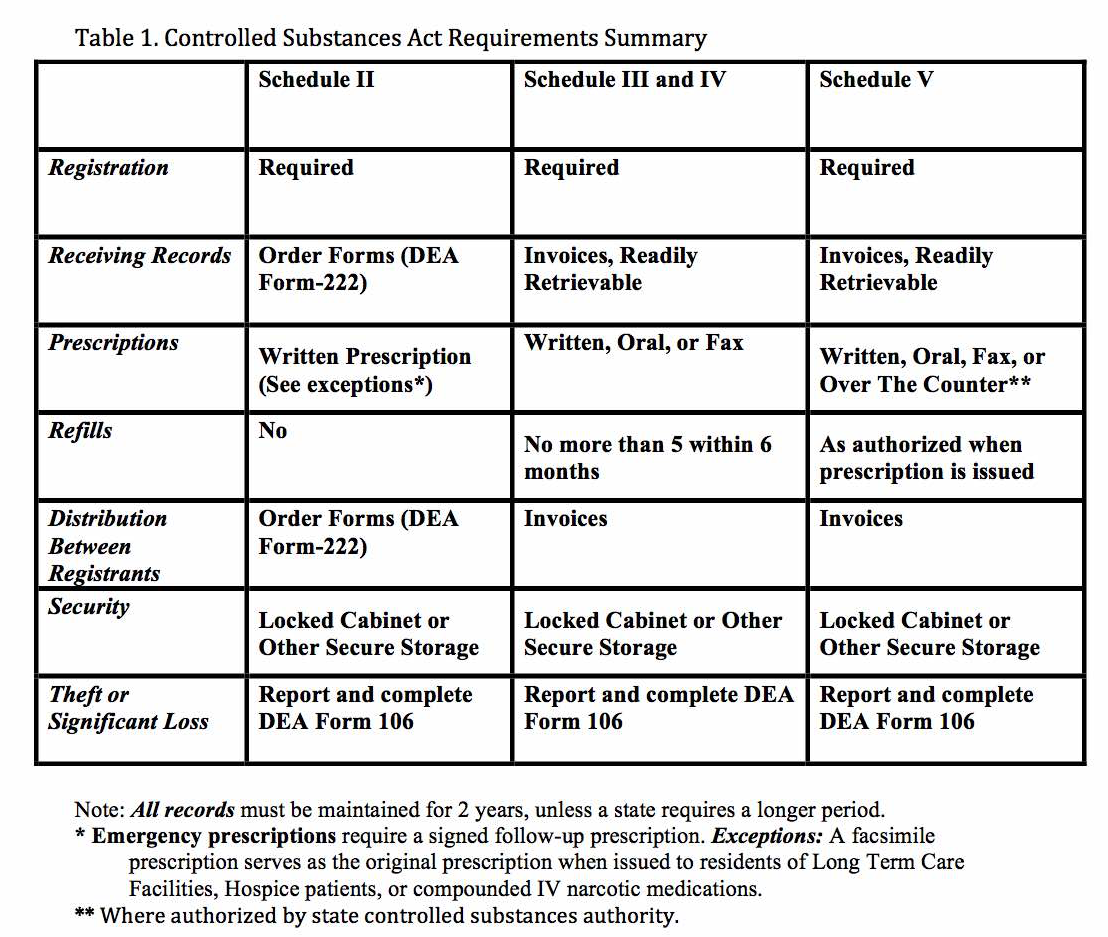
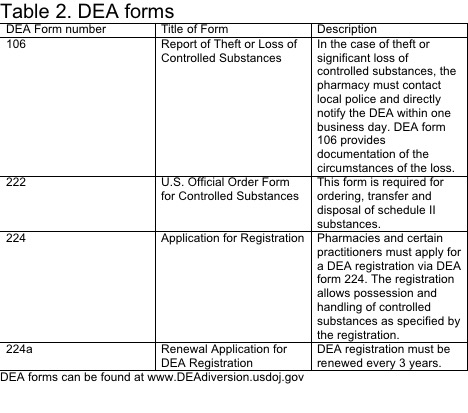
The U.S. Drug Enforcement Administration (DEA) sets national standards for controlled substances. Drug scheduling was mandated under The Federal Comprehensive Drug Abuse Prevention and Control Act of 1970. The law addresses controlled substances within Title II. The DEA maintains a list of controlled medications and illicit substances that are categorized from scheduled I to V. The five categories have their basis on the medication’s proper and beneficial medical use and the medication’s potential for dependency and abuse. The purpose of the law is to provide government oversight over the manufacturing and distribution of these types of substances. Prescribers and dispensers are required to have a DEA license to supply these drugs. The licensing provides links to users, prescribers, and distributors.[120][121][122]
The schedules range from Schedule I to V. Schedule I drugs are considered to have the highest risk of abuse while Schedule V drugs have the lowest potential for abuse. Other factors considered by the DEA include pharmacological effect, evidenced-based knowledge of the drug, risk to public health, trends in the use of the drug, and whether or not the drug has the potential to be made more dangerous with minor chemical modifications.
| Schedule | |
| I |
|
| II |
|
| III |
|
| IV |
|
| V |
|
It is essential to understand the DEA controlled-substance scheduling both to ensure adequate caution when prescribing medications with high abuse potential and also to ensure against prescribing outside of one's authority.[123][124]
The Controlled Substances Act has great potential to improve patient safety by providing federal oversight for drugs with a high potential for abuse. Providers of scheduled substances (physicians, dentists, podiatrists, advanced practitioners) may have links to the distribution of these substances. They are required to have a DEA license and record prescription of scheduled drugs. This licensing prevents overprescribing and obligates providers to be wary of potential drug-seeking patients. The dispenser must also be aware of a patient's medication history and be mindful of the potential for polypharmacy if a patient seeks multiple providers. The current opioid epidemic is a time where federal oversight and interdisciplinary coordination have the potential to reduce harm to patients prescribed scheduled drugs drastically. It will, however, take further time and evaluation to know if drug scheduling actually reduces abuse, addiction, and overdose.[125][126][127][128][129]
See Table 1 for information regarding registration, records, prescriptions, refills, distribution, security, and theft or significant loss of controlled substances.
See Table 2 for information regarding DEA forms 106, 222, 224, and 224a.
West Virginia State Laws
The prescribing, dispensing, reporting, and consumption of controlled substances is governed by West Virginia Code Chapter 60A (Appendix). A brief summary of Virginia State Laws is provided in the following table. Please review the entire law prior to prescribing controlled substances.
| Bill | Summary |
| 81 |
|
| 273 |
The Opioid Reduction Act
|
| 335 |
|
| 362 |
|
| 365 |
|
| 437 |
|
| 514 |
|
| 627 |
|
| 2620 |
|
West Virginia Controlled Substance Monitoring Program
West Virginia has enacted controlled substance monitoring programs to facilitate the collection, analysis, and reporting of controlled substances prescribing and dispensing. The WV program requires:
- All clinicians who prescribe/dispense pain-relieving substances to register with the West Virginia Controlled Substances Monitoring Program database within 30 days of licensure at https://www.csappwv.com
- At least annually accessing the system for specific patients for whom they are providing controlled substances for chronic, nonmalignant pain not due to terminal illness
- Controlled Substances Monitoring Program may be queried prior to accepting a new patient in order to determine whether or not to accept the patient and provide treatment
- If relevant, practitioners may obtain information regarding a breastfeeding mother of a child patient
Health professionals who dispense Schedule II, III and IV controlled substances must provide the dispensing information to the West Virginia Board of Pharmacy each 24-hour period through the Controlled Substances Automated Prescription Program (CSAPP). Pharmacists and approved officers of law enforcement agencies who enforce prescription drug laws can also register for a CSAPP account. All patient information is kept confidential in compliance with HIPAA Privacy and Security Rules. User access is limited to seeking information only in the care of current patients.
The West Virginia Uniform Controlled Substances Act provides the basis for the rules and regulations concerning opioids. The key articles are provided. Providers should review the act before they start prescribing controlled substances.
West Virginia Code 60A: Uniform Controlled Substance Act
ARTICLE 1. DEFINITIONS.
§60A-1-101. Definitions.
As used in this act:
(a) “Administer” means the direct application of a controlled substance whether by injection, inhalation, ingestion, or any other means to the body of a patient or research subject by:
(1) A practitioner (or, in his or her presence, by his or her authorized agent); or
(2) The patient or research subject at the direction and in the presence of the practitioner.
(b) “Agent” means an authorized person who acts on behalf of or at the direction of a manufacturer, distributor, or dispenser. It does not include a common or contract carrier, public warehouseman, or employee of the carrier or warehouseman.
(c) “Analogue” means a substance that, in relation to a controlled substance, has a substantially similar chemical structure.
(d) “Bureau” means the “Bureau of Narcotics and Dangerous Drugs, United States Department of Justice” or its successor agency.
(e) “Controlled substance” means a drug, substance, or immediate precursor in Schedules I through V of article two of this chapter.
(f) “Counterfeit substance” means a controlled substance which, or the container or labeling of which, without authorization, bears the trademark, trade name or other identifying mark, imprint, number or device, or any likeness thereof, of a manufacturer, distributor or dispenser other than the person who in fact manufactured, distributed or dispensed the substance.
(g) “Imitation controlled substance” means: (1) A controlled substance which is falsely represented to be a different controlled substance; (2) a drug or substance which is not a controlled substance but which is falsely represented to be a controlled substance; or (3) a controlled substance or other drug or substance or a combination thereof which is shaped, sized, colored, marked, imprinted, numbered, labeled, packaged, distributed or priced so as to cause a reasonable person to believe that it is a controlled substance.
(h) “Deliver” or “delivery” means the actual, constructive, or attempted transfer from one person to another of (1) A controlled substance, whether or not there is an agency relationship; (2) a counterfeit substance; or (3) an imitation controlled substance.
(i) “Dispense” means to deliver a controlled substance to an ultimate user or research subject by or pursuant to the lawful order of a practitioner, including the prescribing, administering, packaging, labeling, or compounding necessary to prepare the substance for that delivery.
(j) “Dispenser” means a practitioner who dispenses.
(k) “Distribute” means to deliver, other than by administering or dispensing, a controlled substance, a counterfeit substance, or an imitation controlled substance.
(l) “Distributor” means a person who distributes.
(m) “Drug” means (1) Substances recognized as drugs in the official “United States Pharmacopoeia, official Homeopathic Pharmacopoeia of the United States or official National Formulary”, or any supplement to any of them; (2) substances intended for use in the diagnosis, cure, mitigation, treatment or prevention of disease in man or animals; (3) substances (other than food) intended to affect the structure or any function of the body of man or animals; and (4) substances intended for use as a component of any article specified in subdivision (1), (2) or (3) of this subdivision. It does not include devices or their components, parts, or accessories.
(n) “Fentanyl analog or derivative” means any substance which has a chemical structure that is substantially similar to the chemical structure of fentanyl, including any of its salts, isomers, or salts of isomers, including any chemical compound or mixture. For purposes of this chapter, the term “fentanyl derivative or analog” includes any fentanyl analog that is not otherwise scheduled in this chapter.
(o) “Immediate derivative” means a substance that is the principal compound or any analog of the parent compound manufactured from a known controlled substance primarily for use and which has equal or similar pharmacologic activity as the parent compound which is necessary to prevent, curtail or limit manufacture.
(p) “Immediate precursor” means a substance which is the principal compound commonly used or produced primarily for use and which is an immediate chemical intermediary used or likely to be used in the manufacture of a controlled substance, the control of which is necessary to prevent, curtail or limit manufacture.
(q) “Manufacture” means the production, preparation, propagation, compounding, conversion, or processing of a controlled substance, either directly or indirectly or by extraction from substances of natural origin, or independently by means of chemical synthesis, or by a combination of extraction and chemical synthesis, and includes any packaging or repackaging of the substance or labeling or relabeling of its container, except that this term does not include the preparation, compounding, packaging or labeling of a controlled substance:
(1) By a practitioner as an incident to his or her administering or dispensing of a controlled substance in the course of his or her professional practice; or
(2) By a practitioner, or by his or her authorized agent under his or her supervision, for the purpose of, or as an incident to, research, teaching, or chemical analysis and not for sale.
(r) “Marijuana” means all parts of the plant “Cannabis sativa L.”, whether growing or not; the seeds thereof; the resin extracted from any part of the plant; and every compound, manufacture, salt, immediate derivative, mixture, or preparation of the plant, its seeds or resin. It does not include the mature stalks of the plant, fiber produced from the stalks, oil or cake made from the seeds of the plant, any other compound, manufacture, salt, immediate derivative, mixture, or preparation of the mature stalks (except the resin extracted therefrom), fiber, oil or cake, or the sterilized seed of the plant which is incapable of germination.
(s) “Narcotic drug” means any of the following, whether produced directly or indirectly by extraction from substances of vegetable origin or independently by means of chemical synthesis, or by a combination of extraction and chemical synthesis:
(1) Opium and opiate and any salt, compound, immediate derivative, or preparation of opium or opiate.
(2) Any salt, compound, isomer, immediate derivative, or preparation thereof which is chemically equivalent or identical with any of the substances referred to in paragraph (1) of this subdivision, but not including the isoquinoline alkaloids of opium.
(3) Opium poppy and poppy straw.
(4) Coca leaves and any salt, compound, immediate derivative or preparation of coca leaves and any salt, compound, isomer, immediate derivative or preparation thereof which is chemically equivalent or identical with any of these substances, but not including decocainized coca leaves or extractions of coca leaves which do not contain cocaine or ecgonine.
(t) “Opiate” means any substance having an addiction-forming or addiction-sustaining liability similar to morphine or being capable of conversion into a drug having addiction-forming or addiction-sustaining liability. It does not include unless specifically designated as controlled under section two hundred one, article two of this chapter, the dextrorotatory isomer of 3-methoxy-n-methylmorphinan and its salts (dextromethorphan). It does not include its racemic and levorotatory forms.
(u) “Opium poppy” means the plant of the species “Papaver somniferum L.”, except its seeds.
(v) “Person” means an individual, corporation, government or governmental subdivision or agency, business trust, estate, trust, partnership or association, or any other legal entity.
(w) “Placebo” means an inert medicament or preparation administered or dispensed for its psychological effect, to satisfy a patient or research subject or to act as a control in experimental series.
(x) “Poppy straw” means all parts, except the seeds, of the opium poppy after mowing.
(y) “Practitioner” means:
(1) A physician, dentist, veterinarian, scientific investigator, or other person licensed, registered, or otherwise permitted to distribute, dispense, conduct research with respect to, or to administer a controlled substance in the course of professional practice or research in this state.
(2) A pharmacy, hospital, or other institution licensed, registered, or otherwise permitted to distribute, dispense, conduct research with respect to, or to administer a controlled substance in the course of professional practice or research in this state.
(z) “Production” includes the manufacture, planting, cultivation, growing, or harvesting of a controlled substance.
(aa) “State”, when applied to a part of the United States, includes any state, district, commonwealth, territory, insular possession thereof, and any area subject to the legal authority of the United States of America.
(bb) “Ultimate user” means a person who lawfully possesses a controlled substance for his or her own use or for the use of a member of his or her household or for administering to an animal owned by him or her or by a member of his or her household.
ARTICLE 2. STANDARDS AND SCHEDULES.
§60A-2-201. Authority of Board of Pharmacy; recommendations to Legislature.
(a) The Board of Pharmacy shall administer the provisions of this chapter. It shall also, on the first day of each regular legislative session, recommend to the Legislature which substances should be added to or deleted from the schedules of controlled substances contained in this article or reschedule therein. The Board of Pharmacy shall also have the authority between regular legislative sessions, on an emergency basis, to add to or delete from the schedules of controlled substances contained in this article or reschedule such substances based upon the recommendations and approval of the federal food, drug and cosmetic agency, and shall report such actions on the first day of the regular legislative session immediately following said actions.
In making any such recommendation regarding a substance, the Board of Pharmacy shall consider the following factors:
(1) The actual or relative potential for abuse;
(2) The scientific evidence of its pharmacological effect, if known;
(3) The state of current scientific knowledge regarding the substance;
(4) The history and current pattern of abuse;
(5) The scope, duration, and significance of abuse;
(6) The potential of the substance to produce psychic or physiological dependence liability; and
(7) Whether the substance is an immediate precursor of a substance already controlled under this article.
(b) After considering the factors enumerated in subsection (a), the Board of Pharmacy shall make findings with respect to the substance under consideration. If it finds that any substance not already controlled under any schedule has a potential for abuse, it shall recommend to the Legislature that the substance be added to the appropriate schedule. If it finds that any substance already controlled under any schedule should be rescheduled or deleted, it shall so recommend to the Legislature.
(c) If the Board of Pharmacy designates a substance as an immediate precursor, substances which are precursors of the controlled precursor shall not be subject to control solely because they are precursors of the controlled precursor.
(d) If any substance is designated, rescheduled, or deleted as of a controlled substance under federal laws and notice thereof is given to the Board of Pharmacy, the board shall recommend similar control of such substance to the Legislature, specifically stating that such recommendation is based on federal action and the reasons why the federal government deemed such action necessary and proper.
(e) The authority vested in the board by subsection (a) of this section shall not extend to distilled spirits, wine, malt beverages, or tobacco as those terms are defined or used in other chapters of this code nor to any nonnarcotic substance if such substance may under the “Federal Food, Drug and Cosmetic Act” and the law of this state lawfully be sold over the counter without a prescription.
(f) Notwithstanding any provision of this chapter to the contrary, the sale, wholesale, distribution, or prescribing of cannabidiol or nabiximols in a product approved by the Food and Drug Administration is permitted and shall be placed on the schedule or descheduled as provided for by the Drug Enforcement Administration.
§60A-2-202. Nomenclature.
The controlled substances listed in the schedules in this article are included by whatever official, common, usual, chemical, or trade name designated.
§60A-2-203. Schedule I criteria.
The state Board of Pharmacy shall recommend to the Legislature that a substance be included in Schedule I if it finds that the substance:
(1) Has a high potential for abuse; and
(2) Has no accepted medical use in treatment in the United States or lacks accepted safety for use in treatment under medical supervision.
§60A-2-204. Schedule I.
(a) Schedule I shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section including their isomers, esters, ethers, salts, and salts of isomers, esters and ethers, whenever the existence of such isomers, esters, ethers, and salts is possible within the specific chemical designation.
(b) Opiates.
Acetyl-alpha-methylfentanyl (N-[1-(1-methyl-2-phenethyl) -4-piperidinyl]—phenylacetamide);
Acetylmethadol;
Allylprodine;
Alphacetylmethadol (except levoalphacetylmethadol also known as levo-alpha-acetylmethadol, levomethadyl acetate, or LAAM);
Alphameprodine;
Alphamethadol;
Alpha-methylfentanyl (N-[1-(alpha-methyl-beta-phenyl) ethyl-4-piperidyl] propionanilide; 1-(1-methyl-2-phenylethyl)-4-(( propanilido) piperidine);
Alpha-methylthiofentanyl (N-[1-methyl-2-(2-thienyl) ethyl- 4-piperidinyl]—phenylpropanamide);
Benzethidine;
Betacetylmethadol;
Beta-hydroxyfentanyl (N-[1-(2-hydroxy-2-phenethyl) -4- piperidinyl]-N-phenylpropanamide);
Beta-hydroxy-3-methylfentanyl (other name: N-[1-(2- hydroxy-2-phenethyl)-3-methyl-4-piperidinyl]-N-phenylpropanamide);
Betameprodine;
Betamethadol;
Betaprodine;
Clonitazene;
Dextromoramide;
Diampromide;
Diethylthiambutene;
Difenoxin;
Dimenoxadol;
Dimepheptanol;
Dimethylthiambutene;
Dioxaphetyl butyrate;
Dipipanone;
Ethylmethylthiambutene;
Etonitazene;
Etoxeridine;
Furethidine;
Hydroxypethidine;
Ketobemidone;
Levomoramide;
Levophenacylmorphan;
3-Methylfentanyl (N-[3-methyl-1-(2-phenylethyl)-4- piperidyl]-N-phenylpropanamide);
3-methylthiofentanyl (N-[3-methyl-1-(2-thienyl) ethyl-4- piperidinyl]—phenylpropanamide);
Morpheridine;
MPPP (1-methyl-4-phenyl-4-propionoxypiperidine);
Noracymethadol;
Norlevorphanol;
Normethadone;
Norpipanone;
Para-fluorofentanyl (N-(4-fluorophenyl)-N-[1-(2- phenethyl)-4-piperidinyl] propanamide);
PEPAP(1-(-2-phenethyl)-4-phenyl-4-acetoxypiperidine);
Phenadoxone;
Phenampromide;
Phenomorphan;
Phenoperidine;
Piritramide;
Proheptazine;
Properidine;
Propiram;
Racemoramide;
Thiofentanyl (N-phenyl-N-[1-(2-thienyl)ethyl-4- piperidinyl]-propanamide);
Tilidine;
Trimeperidine.
(c) Opium derivatives:
Acetorphine;
Acetyldihydrocodeine;
Benzylmorphine;
Codeine methyl bromide;
Codeine-N-Oxide;
Cyprenorphine;
Desomorphine;
Dihydromorphine;
Drotebanol;
Etorphine (except HCl Salt);
Heroin;
Hydromorphinol;
Methyldesorphine;
Methyldihydromorphine;
Morphine methyl bromide;
Morphine methyl sulfonate;
Morphine-N-Oxide;
Myrophine;
Nicocodeine;
Nicomorphine;
Normorphine;
Pholcodine;
Thebacon.
(d) Hallucinogenic substances.
Alpha-ethyltryptamine; some trade or other names: etryptamine; Monase; alpha-ethy-1H-indole-3-ethanamine; 3-(2- aminobutyl) indole; alpha-ET; and AET;
4-bromo-2, 5-dimethoxy-amphetamine; some trade or other names: 4-bromo-2,5-dimethoxy-alpha-methylphenethylamine; 4-bromo- 2,5-DMA;
4-Bromo-2,5-dimethoxyphenethylamine; some trade or other names: 2-(4-bromo-2,5-dimethoxyphenyl)-1-aminoethane; alpha- desmethyl DOB; 2C-B, Nexus;
N-(2-Methoxybenzyl)-4-bromo-2, 5-dimethoxyphenethylamine. The substance has the acronym 25B-NBOMe.
2-(4-chloro-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl) ethanamine (25C-NBOMe)
2-(4-iodo-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl) ethanamine (25I-NBOMe)
2,5-dimethoxyamphetamine; some trade or other names: 2,5-dimethoxy-alpha-methylphenethylamine; 2,5-DMA;
2,5-dimethoxy-4-ethylamphet-amine; some trade or other names: DOET;
2,5-dimethoxy-4-(n)-propylthiophenethylamine (other name: 2C-T-7);
4-methoxyamphetamine; some trade or other names: 4-methoxy-alpha-methylphenethylamine; paramethoxyamphetamine; PMA;
5-methoxy-3, 4-methylenedioxy-amphetamine;
4-methyl-2,5-dimethoxy-amphetamine; some trade and other names: 4-methyl-2,5-dimethoxy-alpha-methylphenethylamine; “DOM”; and “STP”;
3,4-methylenedioxy amphetamine;
3,4-methylenedioxymethamphetamine (MDMA);
3,4-methylenedioxy-N-ethylamphetamine (also known as ( ethyl-alpha-methyl-3,4 (methylenedioxy) phenethylamine, N-ethyl MDA, MDE, MDEA);
N-hydroxy-3,4-methylenedioxyamphetamine (also known as ( hydroxy-alpha-methyl-3,4 (methylenedioxy) phenethylamine, and ( hydroxy MDA);
3,4,5-trimethoxy amphetamine;
5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT);
Alpha-methyltryptamine (other name: AMT);
Bufotenine; some trade and other names: 3-(beta-Dimethylaminoethyl)-5-hydroxyindole;3-(2-dimethylaminoethyl) -5-indolol; N, N-dimethylserotonin; 5-hydroxy-N,N- dimethyltryptamine; mappine;
Diethyltryptamine; sometrade and other names: N, N-Diethyltryptamine; DET;
Dimethyltryptamine; some trade or other names: DMT;
5-Methoxy-N,N-diisopropyltryptamine (5-MeO-DIPT);
Ibogaine; some trade and other names: 7-Ethyl-6, 6 Beta, 7, 8, 9, 10, 12, 13-octahydro-2-methoxy-6, 9-methano-5H- pyrido [1’, 2’: 1, 2] azepino [5,4-b] indole; Tabernanthe iboga;
Lysergic acid diethylamide;
Marihuana;
Mescaline;
Parahexyl-7374; some trade or other names: 3-Hexyl -1-hydroxy-7, 8, 9, 10-tetrahydro-6, 6, 9-trimethyl-6H-dibenzo [b,d] pyran; Synhexyl;
Peyote; meaning all parts of the plant presently classified botanically as Lophophora williamsii Lemaire, whether growing or not, the seeds thereof, any extract from any part of such plant, and every compound, manufacture, salts, immediate derivative, mixture or preparation of such plant, its seeds or extracts;
N-ethyl-3-piperidyl benzilate;
N-methyl-3-piperidyl benzilate;
Psilocybin;
Psilocyn;
Tetrahydrocannabinol; synthetic equivalents of the substances contained in the plant, or in the resinous extractives of Cannabis, sp. and/or synthetic substances, immediate derivatives and their isomers with similar chemical structure and pharmacological activity such as the following:
delta-1 Cis or trans tetrahydrocannabinol, and their optical isomers;
delta-6 Cis or trans tetrahydrocannabinol, and their optical isomers;
delta-3,4 Cis or trans tetrahydrocannabinol, and its optical isomers;
(Since nomenclature of these substances is not internationally standardized, compounds of these structures, regardless of numerical designation of atomic positions covered.)
Ethylamine analog of phencyclidine; some trade or other names: N-ethyl-1-phenylcyclohexylamine, (1-phenylcyclohexyl) ethylamine, N-(1-phenylcyclohexyl) ethylamine, cyclohexamine, PCE;
Pyrrolidine analog of phencyclidine; some trade or other names: 1-(1-phenylcyclohexyl)-pyrrolidine, PCPy, PHP;
Thiophene analog of phencyclidine; some trade or other names: 1-[1-(2-thienyl)-cyclohexyl]-piperidine, 2-thienylanalog of phencyclidine; TPCP, TCP;
1[1-(2-thienyl)cyclohexyl]pyrroldine; some other names: TCPy.
4-methylmethcathinone (Mephedrone);
3,4-methylenedioxypyrovalerone (MDPV);
2-(2,5-Dimethoxy-4-ethylphenyl)ethanamine (2C-E);
2-(2,5-Dimethoxy-4-methylphenyl)ethanamine (2C-D)
2-(4-Chloro-2,5-dimethoxyphenyl)ethanamine (2C-C)
2-(4-Iodo-2,5-dimethoxyphenyl)ethanamine (2C-I)
2-[4-(Ethylthio)-2,5-dimethoxyphenyl]ethanamine (2C-T-2)
2-[4-(Isopropylthio)-2,5-dimethoxyphenyl]ethanamine (2C-T-4)
2-(2,5-Dimethoxyphenyl)ethanamine (2C-H)
2-(2,5-Dimethoxy-4-nitro-phenyl)ethanamine (2C-N)
2-(2,5-Dimethoxy-4-(n)-propylphenyl)ethanamine (2C-P)
3,4-Methylenedioxy-N-methylcathinone (Methylone)
2,5-dimethoxy-4-(n)-propyltghiophenethylamine (2C-T-7, itsoptical isomers, salts and salts of isomers
5-methoxy-N,N-dimethyltryptamine some trade or other names: 5-methoxy-3-[2-(dimethylamino)ethyl]indole; 5-MeO-DMT(5-MeO-DMT)
Alpha-methyltryptamine (other name: AMT)
5-methoxy-N,N-diisopropyltryptamine (other name: 5-MeO-DIPT)
Synthetic Cannabinoids as follows:
2-[(1R,3S)-3-hydroxycyclohexyl]-5- (2-methyloctan-2-yl)phenol) {also known as CP 47,497 and homologues};
rel-2-[(1S,3R)-3-hydroxycyclohexyl] -5-(2-methylnonan-2-yl)phenol {also known as CP 47,497-C8 homolog};
[(6aR)-9-(hydroxymethyl)-6, 6-dimethyl-3-(2-methyloctan-2-yl)-6a, 7,10,10a-tetrahydrobenzo[c]chromen-1-ol)] {also known as HU-210};
(dexanabinol);
(6aS,10aS)-9-(hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a,7,10,10a-tetrahydrobenzol[c]chromen-1-ol) {also known as HU-211};
1-Pentyl-3-(1-naphthoyl)indole {also known as JWH-018};
1-Butyl-3-(1-naphthoyl)indole {also known as JWH-073};
(2-methyl-1-propyl-1H-indol-3-yl)-1-napthalenyl-methanone {also known as JWH-015};
(1-hexyl-1H-indol-3-yl)-1-naphthalenyl-methanone {also known as JWH-019};
[1-[2-(4-morpholinyl) ethyl] -1H-indol-3-yl]-1-naphthalenyl-methanone {also known as JWH-200};
1-(1-pentyl-1H-indol-3-yl)-2-(3-hydroxyphenyl)-ethanone {also known as JWH-250};
2-((1S,2S,5S)-5-hydroxy-2- (3-hydroxtpropyl)cyclohexyl) -5-(2-methyloctan-2-yl)phenol {also known as CP 55,940};
(4-methyl-1-naphthalenyl) (1-pentyl-1H-indol-3-yl) -methanone {also known as JWH-122};
(4-methyl-1-naphthalenyl) (1-pentyl-1H-indol-3-yl) -methanone {also known as JWH-398;
(4-methoxyphenyl)(1-pentyl-1H-indol-3-yl)methanone {also known as RCS-4};
1-(1-(2-cyclohexylethyl) -1H-indol-3-yl) -2-(2-methoxyphenyl) ethanone {also known as RCS-8};
1-pentyl-3-[1-(4-methoxynaphthoyl)]indole (JWH-081);
1-(5-fluoropentyl)-3-(1-naphthoyl)indole (AM2201); and
1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole (AM694).
Synthetic cannabinoids:
CP 47,497 AND homologues, 2-[(1R,3S)-3-Hydroxycyclohexyl]-5-(2-methyloctan-2-
YL)phenol);
HU-210, [(6AR,10AR)-9-(hydroxymethyl)-6,6-dimethyl-3-(2-Methyloctan-2-YL)-6A,7,10, 10A-tetrahydrobenzo[C] chromen-1-OL)];
HU-211, (dexanabinol, (6AS,10AS)-9-(hydroxymethyl)-6,6-Dimethyl-3-(2-methyloctan-2-YL)-6A,7,10,10atetrahydrobenzo[ C]chromen-1-OL);
JWH-018, 1-pentyl-3-(1-naphthoyl)indole;
JWH-019, 1-hexyl-3-(1-naphthoyl)indole;
JWH-073, 1-butyl-3-(1-naphthoyl)indole;
JWH-200, (1-(2-morpholin-4-ylethyl)indol-3-yl)- Naphthalen-1-ylmethanone;
JWH-250, 1-pentyl-3-(2-methoxyphenylacetyl)indole.]
Methyl 2-(1-(5-fluoropentyl)-1H-indazole-3-carboxamido)-3,3-dimethylbutanoate (5F-ADB);
Methyl 2-(1-(5-fluoropentyl)-1H-indazole-3-carboxamido)-3-methylbutanoate (5F-AMB);
Methyl 2-(1-(4-fluorobenzyl)-1H-indazole-3-carboxamido)-3-methylbutanoate (FUB-AMB);
N-(adamantan-1-yl)-1-(5-fluoropentyl)-1H-indazole-3-carboxamide (5F-APINACA);
N-(1-amino-3,3-dimethyl-1-oxobutan-2-yl)-1-(4-fluorobenzyl)-1H-indazole-3-carboxamide (ADB-FUBINACA);
Methyl 2-(1-(cyclohexylmethyl)-1H-indole-3-carboxamido)-3,3-dimethylbutanoate (MDMB-CHMICA);
Methyl 2-(1-(4-fluorobenzyl)-1H-indazole-3-carboxamido)-3,3-dimethylbutanoate (MDMB-FUBINACA);
Tetrahydrocannabinols:
DELTA-1 CIS OR trans tetrahydrocannabinol and their Optical isomers.
DELTA-6 CIS OR trans tetrahydrocannabinol and their optical isomers.
DELTA-3,4 CIS or their trans tetrahydrocannabinol and their optical isomers.
Synthetic Phenethylamines
2-(4-iodo-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine (25I-NBOMe/ 2C-I-NBOMe);
2-(4-chloro-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine (25C-NBOMe/2C-C-NBOMe);
2-(4-bromo-2,5-dimethoxyphenyl)-N-(2-methoxybenzyl)ethanamine (25B-NBOMe/ 2C-B-NBOMe);
Synthetic Opioids (icluding their isomers, esters, ethers, salts and salts of isomers, esters and ethers):
N-(1-phenethylpiperidin-4-yl)-N-phenylacetamide (acetyl fentanyl);
furanyl fentanyl;
3,4-dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methylbenzamide (also known as U-47700);
N-(1-phenethylpiperidin-4-yl)-N-phenylbutyramide, also known as N-(1-phenethylpiperidin-4-yl)-N-phenylbutanamide, (butyryl fentanyl);
N-[1-[2-hydroxy-2-(thiophen-2-yl)ethylpiperidin-4-yl]-N-phenylpropionamide, also known as N-[1-[2-hydroxy-2-(2-thienyl)ethyl]-4-piperidinyl]-N-phenylpropanamide, (beta-hydroxythiofentanyl).
N-(1-phenethylpiperidin-4-yl)-N-phenylacrylamide (acryl fentanyl)
N-(1-phenethylpiperidin-4-yl)-N-phenylisobutyramide (isobutyryl fentanyl)
N-(1-phenethylpiperidin-4-yl)-N-phenylcyclopentanecarboxamide (cyclopropyl fentanyl)
2-(2,4-dichlorophenyl)-N-((1S,2S)-2-(dimethylamino)cyclohexyl)-N-methylacetamide (also known as U-48800)
Trans-3,4-dichloro-N-[2-(diethylamino)cyclohexyl]-N-methyl-benzamide (also known as U-49900)
Trans-3,4-dichloro-N-[2-(dimethylamino)cyclohexyl]-N-methyl-benzeneacetamide (also known as U-51754)
Opioid Receptor Agonist
AH-7921 (3,4-dichloro-N- (1dimethylamino)cyclohexylmethyl]benzamide).
Naphthoylindoles or any compound containing a 3-(-1- Napthoyl) indole structure with substitution at the nitrogen atom of the indole ring whether or not further substituted in the indole ring to any extent and whether or not substituted in the naphthyl ring to any extent. This shall include the following:
JWH 015;
JWH 018;
JWH 019;
JWH 073;
JWH 081;
JWH 122;
JWH 200;
JWH 210;
JWH 398;
AM 2201;
WIN 55,212.
Naphylmethylindoles or any compound containing a 1hindol-3-yl-(1-naphthyl) methane structure with a substitution at the nitrogen atom of the indole ring whether or not further substituted in the indole ring to any extent and whether or not substituted in the naphthyl ring to any extent. This shall include, but not be limited to, JWH 175 and JWH 184.
Naphthoylpyrroles or any compound containing a 3-(1- Naphthoyl) pyrrole structure with substitution at the nitrogen atom of the pyrrole ring whether or not further substituted in the pyrrole ring to any extent and whether or not substituted in the naphthyl ring to any extent. This shall include, but not be limited to, JWH 147 and JWH 307.
Naphthylmethylindenes or any compound containing a Naphthylideneindene structure with substitution at the 3- Position of the indene ring whether or not further substituted in the indene ring to any extent and whether or not substituted in the naphthyl ring to any extent. This shall include, but not be limited to, JWH 176.
Phenylacetylindoles or any compound containing a 3- Phenylacetylindole structure with substitution at the nitrogen atom of the indole ring whether or not further substituted in the indole ring to any extent and whether or not substituted in the phenyl ring to any extent. This shall include the following:
RCS-8, SR-18 OR BTM-8;
JWH 250;
JWH 203;
JWH 251;
JWH 302.
Cyclohexylphenols or any compound containing a 2-(3- hydroxycyclohexyl) phenol structure with a substitution at the 5-position of the phenolic ring whether or not substituted in the cyclohexyl ring to any extent. This shall include the following:
CP 47,497 and its homologues and analogs;
Cannabicyclohexanol;
CP 55,940.
Benzoylindoles or any compound containing a 3-(benzoyl) indole structure with substitution at the nitrogen atom of the indole ring whether or not further substituted in the indole ring to any extent and whether or not substituted in the phenyl ring to any extent. This shall include the following:
AM 694;
Pravadoline WIN 48,098;
RCS 4;
AM 679.
[2,3-dihydro-5 methyl-3-(4-morpholinylmethyl)pyrrolo [1,2,3-DE]-1, 4-benzoxazin-6-YL]-1-napthalenymethanone. This shall include WIN 55,212-2.
Dibenzopyrans or any compound containing a 11-hydroxydelta 8-tetrahydrocannabinol structure with substitution on the 3-pentyl group. This shall include HU-210, HU-211, JWH 051 and JWH 133.
Adamantoylindoles or any compound containing a 3-(-1- Adamantoyl) indole structure with substitution at the nitrogen atom of the indole ring whether or not further substituted in the adamantoyl ring system to any extent. This shall include AM1248.
Tetramethylcyclopropylindoles or any compound containing A 3-tetramethylcyclopropylindole structure with substitution at the nitrogen atom of the indole ring whether or not further substituted in the indole ring to any extent and whether or not substituted in the tetramethylcyclopropyl ring to any extent. This shall include UR-144 and XLR-11.
N-(1-Adamantyl)-1-pentyl-1h-indazole-3-carboxamide. This shall include AKB48.
Any other synthetic chemical compound that is a Cannabinoid receptor type 1 agonist as demonstrated by binding studies and functional assays that is not listed in Schedules II, III, IV and V, not federal Food and Drug Administration approved drug or used within legitimate, approved medical research. Since nomenclature of these substances is not internationally standardized, any immediate precursor or immediate derivative of these substances shall be covered.
Tryptamines:
5- methoxy- N- methyl-N-isopropyltryptamine (5-MeO-MiPT)
4-hydroxy-N,N-diisopropyltryptamine (4-HO-DiPT)
4-hydroxy-N-methyl-N-isopropyltryptamine (4-HO-MiPT)
4-hydroxy-N-methyl-N-ethyltryptamine (4-HO-MET)
4-acetoxy-N,N-diisopropyltryptamine (4-AcO-DiPT)
5-methoxy-α-methyltryptamine (5-MeO-AMT)
4-methoxy-N,N-Dimethyltryptamine (4-MeO-DMT)
4-hydroxy Diethyltryptamine (4-HO-DET)
5- methoxy- N,N- diallyltryptamine (5-MeO-DALT)
4-acetoxy-N,N-Dimethyltryptamine (4-AcO DMT)
4-hydroxy Diethyltryptamine (4-HO-DET)
(e) Depressants.
Mecloqualone;
Methaqualone.
(f) Stimulants.
Aminorex; some other names: aminoxaphen; 2-amino-5- phenyl-2-oxazoline; or 4,5-dihydro-5-phenyl-2-oxazolamine;
Cathinone; some trade or other names: 2-amino-1-phenyl-1- propanone, alpha-aminopropiophenone, 2-aminopropiophenone and norephedrone;
Fenethylline;
Methcathinone, its immediate precursors and immediate derivatives, its salts, optical isomers and salts of optical isomers; some other names: (2-(methylamino)-propiophenone; alpha-
(methylamino)propiophenone; 2-(methylamino)-1-phenylpropan-1- one; alpha—-methylaminopropiophenone; monomethylpropion; 3,4-methylenedioxypyrovalerone and/or mephedrone;3,4-methylenedioxypyrovalerone (MPVD); ephedrone; N-methylcathinone; methylcathinone; AL-464; AL-422; AL- 463 and UR1432;
(+-) cis-4-methylaminorex; ((+-)cis-4,5-dihydro-4-methyl- 5-phenyl-2-oxazolamine);
N-ethylamphetamine;
N,N-dimethylamphetemine; also known as N,N-alpha- trimethyl-benzeneethanamine; N,N-alpha-trimethylphenethylamine.
Alpha-pyrrolidinopentiophenone, also known as alpha-PVP, optical isomers, salts and salts of isomers.
Substituted amphetamines:
2-Fluoroamphetamine
3-Fluoroamphetamine
4-Fluoroamphetamine
2-chloroamphetamine
3-chloroamphetamine
4-chloroamphetamine
2-Fluoromethamphetamine
3-Fluoromethamphetamine
4-Fluoromethamphetamine
4-chloromethamphetamine
(g) Temporary listing of substances subject to emergency scheduling. Any material, compound, mixture, or preparation which contains any quantity of the following substances:
N-[1-benzyl-4-piperidyl]-N-phenylpropanamide (benzylfentanyl), its optical isomers, salts, and salts of isomers.
N-[1-(2-thienyl)methyl-4-piperidyl]-N-phenylpropanamide (thenylfentanyl), its optical isomers, salts, and salts of isomers.
N-benzylpiperazine, also known as BZP.
Cyclopentyl fentanyl (N-(1-phenethylpiperidin-4-yl)-N-phenylcyclopentanecarboxamide);
4-fluorobutyryl fentanyl (N-(4-fluorophenyl)-N-[1-(2-phenylethyl)piperidin-4-yl]-butyramide);
Isobutyryl fentanyl (2-methyl-N-phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]-propanamide);
Methoxyacetyl fentanyl (2-methoxy-N-phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]-acetamide);
3-methylbutyryl fentanyl (N-[3-methyl-1-(2-phenylethyl)piperidin-4-yl]-N-phenylbutyramide);
4-methoxybutyryl fentanyl (N-(4-methoxyphenyl)-N-(1-phenethylpiperidin-4-yl)butyramide);
Ocfentanil (N-(2-fluorophenyl)-2-methoxy-N-[1-(2-phenylethyl)piperidin-4-yl]-acetamide);
Tetrahydrofuran fentanyl (N-(1-phenethylpiperidin-4-yl)-N-phenyltetrahydrofuran-2-carboxamide);
Valeryl fentanyl (N-phenyl-N-[1-(2-phenylethyl)piperidin-4-yl]pentanamide).
(h) The following controlled substances are included in Schedule I:
Synthetic Cathinones or any compound, except bupropion or compounds listed under a different schedule, or compounds used within legitimate and approved medical research, structurally derived from 2- Aminopropan-1-one by substitution at the 1-position with Monocyclic or fused polycyclic ring systems, whether or not the compound is further modified in any of the following ways:
By substitution in the ring system to any extent with Alkyl, alkylenedioxy, alkoxy, haloalkyl, hydroxyl, or halide Substituents whether or not further substituted in the ring system by one or more other univalent substituents.
By substitution at the 3-position with an acyclic alkyl substituent.
By substitution at the 2-amino nitrogen atom with alkyl, dialkyl, benzyl, or methoxybenzyl groups.
By inclusion of the 2-amino nitrogen atom in a cyclic structure.
Any other synthetic chemical compound that is a Cannabinoid receptor type 1 agonist as demonstrated by binding studies and functional assays that is not listed in Schedules II, III, IV, and V, not federal Food and Drug Administration approved drug or used within legitimate, approved medical research.
§60A-2-205. Schedule II criteria.
The state Board of Pharmacy shall recommend to the Legislature that a substance be placed in Schedule II if it finds that:
(1) The substance has a high potential for abuse;
(2) The substance has currently accepted medical use in treatment in the United States or currently accepted medical use with severe restrictions;
(3) Abuse of the substance may lead to severe psychic or physical dependence.
§60A-2-206. Schedule II.
(a) Schedule II consists of the drugs and other substances, by whatever official name, common or usual name, chemical name or brand name designated, listed in this section. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture or preparation which contains any quantity of the following substances, including their isomers, esters, ethers, salts and salts of isomers, esters and ethers, whenever the existence of such isomers, esters, ethers, and salts is possible within the specific chemical designation.
(b) Substances, vegetable origin or chemical synthesis. — Unless specifically excepted or unless listed in another schedule, any of the following substances whether produced directly or indirectly by extraction from substances of vegetable origin, or independently by means of chemical synthesis, or by a combination of extraction and chemical synthesis:
Opium and opiate, and any salt, compound, derivative or preparation of opium or opiate excluding apomorphine, thebaine-derived butorphanol, dextrorphan, nalbuphine, nalmefene, naloxone and naltrexone, and their respective salts, but including the following:
Raw opium;
Opium extracts;
Opium fluid;
Powdered opium;
Granulated opium;
Tincture of opium;
Codeine;
Dihydroetorphine;
Ethylmorphine;
Etorphine hydrochloride;
Hydrocodone;
Hydromorphone;
Metopon;
Morphine;
Oripavine;
Oxycodone;
Oxymorphone; and
Thebaine;
Any salt, compound, derivative or preparation thereof which is chemically equivalent or identical with any of the substances referred to in subdivision (1) of this subsection, except that these substances shall not include the isoquinoline alkaloids of opium;
Opium poppy and poppy straw;
Coca leaves and any salt, compound, derivative, or preparation of coca leaves (including cocaine and ecgonine and their salts, isomers, derivatives, and salts of isomers and derivatives), and any salt, compound, derivative, or preparation thereof which is chemically equivalent or identical with any of these substances, except that the substances shall not include decocainized coca leaves or extractions of coca leaves, which extractions do not contain cocaine or ecgonine;
Concentrate of poppy straw (the crude extract of poppy straw in either liquid, solid, or powder form which contains the phenanthrene alkaloids of the opium poppy).
(c) Opiates. —
Alfentanil;
Alphaprodine;
Anileridine;
Bezitramide;
Bulk dextropropoxyphene (nondosage forms);
Carfentanil;
Dihydrocodeine;
Diphenoxylate;
Fentanyl;
Isomethadone;
Levo-alphacetylmethadol; some other names: levo-alpha-acetylmethadol, levomethadyl acetate, LAAM;
Levomethorphan;
Levorphanol;
Metazocine;
Methadone;
Methadone-Intermediate, 4-cyano-2-dimethylamino-4, 4-diphenyl butane;
Moramide-Intermediate, 2-methyl-3-morpholino-1,
1-diphenylpropane-carboxylic acid;
Pethidine; (meperidine);
Pethidine-Intermediate-A, 4-cyano-1-methyl-4- phenylpiperidine;
Pethidine-Intermediate-B, ethyl-4-phenylpiperidine-4-carboxylate;
Pethidine-Intermediate-C, 1-methyl-4-phenylpiperidine-4-carboxylic acid;
Phenazocine;
Piminodine;
Racemethorphan;
Racemorphan;
Remifentanil;
Sufentanil;
Tapentadol
Thiafentanil (4-(methoxycarbonyl)-4-(N-phenmethoxyacetamido)-1-2-(thienyl)ethylpiperidine), including its isomers, esters, ethers, salts and salts of isomers, esters and ethers.
(d) Stimulants. —
Amphetamine, its salts, optical isomers, and salts of its optical isomers;
Methamphetamine, its salts, isomers, and salts of its isomers;
Methylphenidate;
Phenmetrazine and its salts; and
Lisdexamfetamine.
(e) Depressants. —
Amobarbital;
Glutethimide;
Pentobarbital;
Phencyclidine;
Secobarbital.
(f) Hallucinogenic substances:
Dronabinol [(-)-delta-9-trans tetrahydrocannabinol] if in an FDA approved oral solution
Nabilone: [Another name for nabilone: (+-)-trans-3-(1, 1-dimethylheptyl)-6, 6a, 7, 8, 10, 10a-hexahydro-1-hydroxy-6, 6-dimethyl-9H-dibenzo [b,d] pyran-9-one].
(g) Immediate precursors. — Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation which contains any quantity of the following substances:
Immediate precursor to amphetamine and methamphetamine:
Phenylacetone;
Some trade or other names: phenyl-2-propanone; P2P; benzyl methyl ketone; methyl benzyl ketone;
Immediate precursors to phencyclidine (PCP):
1-phenylcyclohexylamine; and
1-piperidinocyclohexanecarbonitrile (PCC).
Immediate precursor to fentanyl:
4-anilino-N-phenethyl-4-piperidine (ANPP).
§60A-2-207. Schedule III criteria.
The state Board of Pharmacy shall recommend to the Legislature that a substance be placed in Schedule III if it finds that:
(1) The substance has a potential for abuse less than the substances listed in Schedules I and II;
(2) The substance has currently accepted medical use in treatment in the United States; and
(3) Abuse of the substance may lead to moderate or low physical dependence or high psychological dependence.
§60A-2-208. Schedule III.
(a) Schedule III consists of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section.
(b) Stimulants. -- Unless specifically excepted or unless listed in another schedule, any material, compound, mixture or preparation which contains any quantity of the following substances having a stimulant effect on the central nervous system, including its salts, isomers (whether optical, position, or geometric) and salts of such isomers whenever the existence of the salts, isomers, and salts of isomers is possible within the specific chemical designation:
(1) Those compounds, mixtures or preparations in dosage unit form containing any stimulant substances listed in Schedule II which compounds, mixtures or preparations were listed on August 25, 1971, as excepted compounds under 21 C.F.R. §1308.32, and any other drug of the quantitative composition shown in that list for those drugs or which is the same except that it contains a lesser quantity of controlled substances;
(2) Benzphetamine;
(3) Chlorphentermine;
(4) Clortermine;
(5) Phendimetrazine.
(c) Depressants. -- Unless specifically excepted or unless listed in another schedule, any material, compound, mixture or preparation which contains any quantity of the following substances having a depressant effect on the central nervous system:
(1) Any compound, mixture, or preparation containing:
(A) Amobarbital;
(B) Secobarbital;
(C) Pentobarbital; or any salt of pentobarbital and one or more other active medicinal ingredients which are not listed in any schedule;
(2) Any suppository dosage form containing:
(A) Amobarbital;
(B) Secobarbital;
(C) Pentobarbital; or any salt of any of these drugs and approved by the food and drug administration for marketing only as a suppository;
(3) Any substance which contains any quantity of a derivative of barbituric acid or any salt of barbituric acid;
(4) Aprobarbital;
(5) Butabarbital (secbutabarbital);
(6) Butalbital (including, but not limited to, Fioricet);
(7) Butobarbital (butethal);
(8) Chlorhexadol;
(9) Embutramide;
(10) Gamma Hydroxybutryic Acid preparations;
(11) Ketamine, its salts, isomers and salts of isomers [Some other names for ketamine: (+-)-2-(2-chlorophenyl)-2-(methylamino)-cyclohexanone];
(12) Lysergic acid;
(13) Lysergic acid amide;
(14) Methyprylon;
(15) Sulfondiethylmethane;
(16) Sulfonethylmethane;
(17) Sulfonmethane;
(18) Thiamylal;
(19) Thiopental;
(20) Tiletamine and zolazepam or any salt of tiletamine and zolazepam; some trade or other names for a tiletamine-zolazepam combination product: Telazol; some trade or other names for tiletamine: 2-(ethylamino)-2-(2-thienyl)-cyclohexanone; some trade or other names for zolazepam: 4-(2-flurophenyl)-6, 8-dihydro-1, 3, 8-trimethylpyrazolo-[3,4-e] [1,4]-diazepin-7(1H)-one, flupyrazapon; and
(21) Vinbarbital.
(d) Nalorphine.
(e) Narcotic drugs. -- Unless specifically excepted or unless listed in another schedule:
(1) Any material, compound, mixture or preparation containing any of the following narcotic drugs, or their salts calculated as the free anhydrous base or alkaloid, in limited quantities as set forth below:
(A) Not more than 1.8 grams of codeine per 100 milliliters and not more than 90 milligrams per dosage unit, with an equal or greater quantity of an isoquinoline alkaloid of opium;
(B) Not more than 1.8 grams of codeine per 100 milliliters or not more than 90 milligrams per dosage unit, with one or more active, nonnarcotic ingredients in recognized therapeutic amounts;
(C) Not more than 1.8 grams of dihydrocodeine per 100 milliliters and not more than 90 milligrams per dosage unit, with one or more active, nonnarcotic ingredients in recognized therapeutic amounts;
(D) Not more than 300 milligrams of ethylmorphine per 100 milliliters or not more than 15 milligrams per dosage unit, with one or more active, nonnarcotic ingredients in recognized therapeutic amounts;
(E) Not more than 500 milligrams of opium per 100 milliliters or per 100 grams or not more than 25 milligrams per dosage unit, with one or more active, nonnarcotic ingredients in recognized therapeutic amounts;
(F) Not more than 50 milligrams of morphine per 100 milliliters or per 100 grams, with one or more active, nonnarcotic ingredients in recognized therapeutic amounts.
(2) Any material, compound, mixture, or preparation containing buprenorphine or its salts (including, but not limited to, Suboxone).
(f) Anabolic steroids. -- Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation containing any quantity of anabolic steroids, including its salts, isomers, and salts of isomers whenever the existence of the salts of isomers is possible within the specific chemical designation.
(g) Human growth hormones.
(h) Dronabinol (synthetic) in sesame oil and encapsulated in a soft gelatin capsule in a United States food and drug administration approved drug product. (Some other names for dronabinol: (6aR-trans)-6a, 7, 8, 10a- tetrahydro-6, 6, 9-trimethyl-3-pentyl-6H-dibenzo [b,d] pyran-1- ol or (-)-delta-9-(trans)-tetrahydrocannabinol).
(i) Human chorionic gonadotropin, except when used for injection or implantation in cattle or any other nonhuman species and when that use is approved by the Food and Drug Administration.
§60A-2-209. Schedule IV criteria.
The state Board of Pharmacy shall recommend to the Legislature that a substance be placed in Schedule IV if it finds that:
(1) The substance has a low potential for abuse relative to substances in Schedule III;
(2) The substance has currently accepted medical use in treatment in the United States; and
(3) Abuse of the substance may lead to limited physical dependence or psychological dependence relative to the substances in Schedule III.
§60A-2-210. Schedule IV.
(a) Schedule IV shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture or preparation which contains any quantity of the following substances, including their isomers, esters, ethers, salts and salts of isomers, esters and ethers, whenever the existence of such isomers, esters, ethers, and salts is possible within the specific chemical designation.
(b) Narcotic drugs. — Unless specifically excepted or unless listed in another schedule, any material, compound, mixture, or preparation containing any of the following narcotic drugs, or their salts calculated as the free anhydrous base or alkaloid, in limited quantities as set forth below:
Not more than 1 milligram of difenoxin and not less than 25 micrograms of atropine sulfate per dosage unit;
Dextropropoxyphene (alpha-(+)-4-dimethylamino-1,2-diphenyl-3-methyl-2-propionoxybutane).
(c) Depressants.
Alprazolam;
Barbital;
Bromazepam;
Camazepam;
Carisoprodol;
Chloral betaine;
Chloral hydrate;
Chlordiazepoxide;
Clobazam;
Clonazepam;
Clorazepate;
Clotiazepam;
Cloxazolam;
Delorazepam;
Diazepam;
Dichloralphenazone;
Estazolam;
Ethchlorvynol;
Ethinamate;
Ethyl loflazepate;
Fludiazepam;
Flunitrazepam;
Flurazepam;
Fospropofol;
Halazepam;
Haloxazolam;
Ketazolam;
Loprazolam;
Lorazepam;
Lormetazepam;
Mebutamate;
Medazepam;
Meprobamate;
Methohexital;
Methylphenobarbital (mephobarbital);
Midazolam;
Nimetazepam;
Nitrazepam;
Nordiazepam;
Oxazepam;
Oxazolam;
Paraldehyde;
Petrichloral;
Phenobarbital;
Pinazepam;
Prazepam;
Quazepam;
Temazepam;
Tetrazepam;
Triazolam;
Zaleplon;
Zolpidem;
Zopiclone’
Suvorexant ([(7R)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl] [5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl]methanone).
(d) Any material, compound, mixture or preparation which contains any quantity of Fenfluramine and Dexfenfluramine.
(e) Stimulants.
Cathine ((+)-norpseudoephedrine);
Diethylpropion;
Fencamfamin;
Fenproporex;
Mazindol;
Mefenorex;
Modafinil;
Pemoline (including organometallic complexes and chelates thereof);
Phentermine;
Pipradrol;
Sibutramine;
SPA ((-)-1-dimethylamino-1,2-diphenylethane);
Eluxadoline (5-[[[(2S)-2-amino-3-[4-aminocarbonyl)-2,6-dimethylphenyl]-1-oxopropyl [(1S)-1-(4-phenyl-1H-imidazol-2-yl)ethyl]amino]methyl]-2-methoxybenzoic acid);
(f) Other substances. —
Pentazocine;
Butorphanol.
Tramadol (2-[(dimethylamino)methyl]-1-(3-methoxyphenyl) cyclohexanol);
Amyl nitrite, butyl nitrite, isobutyl nitrite, and the other organic nitrites are controlled substances and no product containing these compounds as a significant component shall be possessed, bought, or sold other than pursuant to a bona fide prescription or for industrial or manufacturing purposes.
§60A-2-211. Schedule V criteria.
The state Board of Pharmacy shall recommend to the Legislature that a substance be placed in Schedule V if it finds that:
(1) The substance has a low potential for abuse relative to the controlled substances listed in Schedule IV;
(2) The substance has currently accepted medical use in treatment in the United States; and
(3) The substance has limited physical dependence or psychological dependence liability relative to the controlled substances listed in Schedule IV.
§60A-2-212. Schedule V.
(a) Schedule V shall consist of the drugs and other substances, by whatever official name, common or usual name, chemical name, or brand name designated, listed in this section. Unless specifically excepted or unless listed in another schedule, any material, compound, mixture or preparation which contains any quantity of the following substances, including their isomers, esters, ethers, salts and salts of isomers, esters and ethers, whenever the existence of such isomers, esters, ethers, and salts is possible within the specific chemical designation.
(b) Narcotic drugs containing nonnarcotic active medicinal ingredients. Any compound, mixture, or preparation containing any of the following narcotic drugs or their salts calculated as the free anhydrous base or alkaloid in limited quantities as set forth below, which shall include one or more nonnarcotic active medicinal ingredients in sufficient proportion to confer upon the compound, mixture or preparation valuable medicinal qualities other than those possessed by the narcotic drug alone.
Not more than 200 milligrams of codeine per 100 milliliters or per 100 grams;
Not more than 100 milligrams of dihydrocodeine per 100 milliliters or per 100 grams;
Not more than 100 milligrams of ethylmorphine per 100 milliliters or per 100 grams;
Not more than 2.5 milligrams of diphenoxylate and not less than 25 micrograms of atropine sulfate per dosage unit;
Not more than 100 milligrams of opium per 100 milliliters or per 100 grams;
Not more than 0.5 milligrams of difenoxin and not less than 25 micrograms of atropine sulfate per dosage unit.
(c) Stimulants: —
Pyrovalerone.
(d) Any compound, mixture, or preparation containing as its single active ingredient ephedrine, pseudoephedrine or phenylpropanolamine, their salts or optical isomers, or salts of optical isomers, except products which are for pediatric use primarily intended for administration to children under the age of 12: Provided, That neither the offenses set forth in section four hundred one, article four of this chapter, nor the penalties therein, shall be applicable to ephedrine, pseudoephedrine or phenylpropanolamine which shall be subject to the provisions of article ten of this chapter.
(e) Depressants: —
Ezogabine [N-[2-amino-4-94-fluorobenzylamino)-phenyl]-carbamic acid ethyl ester];
Lacosamide [(R)-2-acetoamido- N -benzyl-3-methoxy-propionamide];
Pregabalin [(S)-3-(aminomethyl)-5-methylhexanoic acid]; and
Brivaracetam ((2S)-2-[(4R)-2-oxo-4-propylpyrrolidin-1-yl] butanamide) (also referred to as BRV; UCB-34714; Briviact).
(f) Other substances:
Gabapentin
Pregabalin
§60A-2-213. Review and print of schedules by the board; public information.
The state Board of Pharmacy shall annually review and cause to be printed the schedules contained in this article, which printed schedules shall be made available to the public.
ARTICLE 3. REGULATION OF MANUFACTURE, DISTRIBUTION, AND DISPENSING OF CONTROLLED SUBSTANCES. §60A-3-301. Rules; fees.
The state Board of Pharmacy shall promulgate rules and charge fees relating to the registration and control of the manufacture and distribution of controlled substances within this state, and each department, board, or agency of this state which licenses or registers practitioners authorized to dispense any controlled substance shall promulgate rules and charge fees relating to the registration and control of the dispensing of controlled substances within this state by those practitioners licensed or registered by such department, board, or agency.
The state Board of Pharmacy or the department, board or agency shall collect the following annual registration fees from persons who manufacture, distribute, dispense or conduct research with controlled substances: For registration of a manufacturer, $50; for registration of a wholesaler, $50; for registration of a retailer, $15; for registration of a hospital or clinic, $15; and for registration of a research institution, $5.
§60A-3-302. Registration required; effect of registration; exemptions; waiver; inspections.
(a) Every person who manufactures, distributes, or dispenses any controlled substance within this state or who proposes to engage in the manufacture, distribution, or dispensing of any controlled substance within this state, must obtain annually a registration issued by the state Board of Pharmacy or the appropriate department, board, or agency, as the case may be, as specified in section three hundred one, in accordance with its rules.
(b) Persons registered by said state Board of Pharmacy or said appropriate department, board, or agency, as the case may be, under this act to manufacture, distribute, dispense, or conduct research with controlled substances may possess, manufacture, distribute, dispense, or conduct research with those substances to the extent authorized by their registration and in conformity with the other provisions of this article.
(c) (1) The following persons need not register and may lawfully possess, deliver, or transport into this state-controlled substances under this act:
(A) An agent or employee of any registered manufacturer, distributor, or dispenser of any controlled substance if he is acting in the usual course of his business or employment;
(B) A common or contract carrier or warehouseman, or an employee thereof, whose possession, delivery, or transportation into this state of any controlled substance is in the usual course of a lawful business or employment;
(2) The following persons need not register and may lawfully possess or transport into this state-controlled substances under this act: An ultimate user or a person in possession of any controlled substance pursuant to a lawful order of a practitioner or in lawful possession of a Schedule V substance.
(d) The said state Board of Pharmacy or said appropriate department, board, or agency, as the case may be, may waive by rule the requirement for registration of certain manufacturers, distributors, or dispensers if it finds it consistent with the public health and safety.
(e) A separate registration is required at each principal place of business or professional practice where the applicant manufactures, distributes, or dispenses controlled substances.
(f) The said state Board of Pharmacy or said appropriate department, board, or agency, as the case may be, may inspect the establishment of a registrant or applicant for registration in accordance with the rule of said state Board of Pharmacy or said appropriate department, board, or agency, as the case may be.
§60A-3-303. What applicants to be registered; determination of public interest; rights of registrants.
(a) The State Board of Pharmacy shall register an applicant to manufacture or distribute controlled substances included in Schedules I, II, III, IV, and V unless it determines that the issuance of that registration would be inconsistent with the public interest. In determining the public interest, the state Board of Pharmacy shall consider the following factors:
(1) Maintenance of effective controls against diversion of controlled substances into other than legitimate medical, scientific, or industrial channels;
(2) Compliance with applicable state and local law;
(3) Any convictions of the applicant under any federal or state laws relating to any controlled substance;
(4) Past experience in the manufacture or distribution of controlled substances, and the existence in the applicant's establishment of effective controls against diversion;
(5) Furnishing by the applicant of false or fraudulent material in any application filed under this act;
(6) Suspension or revocation of the applicant's federal registration to manufacture, distribute, or dispense controlled substances as authorized by federal law; and
(7) Any other factors relevant to and consistent with public health and safety.
(b) Registration under subsection (a) does not entitle a registrant to manufacture and distribute controlled substances in Schedule I or II other than those specified in the registration.
(c) Practitioners must be registered to dispense any controlled substances or to conduct research with controlled substances in Schedules II through V if they are authorized to dispense or conduct research under the law of this state. The appropriate department, board, or agency, as specified in section 301, need not require separate registration under this article for practitioners engaging in research with non-narcotic controlled substances in Schedules II through V where the registrant is already registered under this article in another capacity. Practitioners registered under federal law to conduct research with Schedule I substances may conduct research with Schedule I substances within this state upon furnishing the appropriate department, board, or agency evidence of that federal registration.
(d) Compliance by manufacturers and distributors with the provisions of the federal law respecting registration (excluding fees) entitles them to be registered under this act.
§60A-3-304. Suspension or revocation of registration generally.
(a) A registration under section 303 to manufacture, distribute, or dispense a controlled substance may be suspended or revoked by the said state Board of Pharmacy or said appropriate department, board, or agency, as the case may be, upon a finding that the registrant:
(1) Has furnished false or fraudulent material information in any application filed under this act;
(2) Has been convicted of a felony under any state or federal law relating to any controlled substance; or
(3) Has had his federal registration suspended or revoked to manufacture, distribute, or dispense controlled substances.
(b) The said state Board of Pharmacy or said appropriate department, board, or agency, as the case may be, may limit suspension or revocation of a registration to the particular controlled substance with respect to which grounds for suspension or revocation exist.
(c) If the said state Board of Pharmacy or said appropriate department, board, or agency, as the case may be, suspends or revokes a registration, all controlled substances owned or possessed by the registrant at the time of suspension or the effective date of the revocation order may be placed under seal. No disposition may be made of substances under seal until the time for taking an appeal has elapsed or until all appeals have been concluded unless a court, upon application therefor, orders the sale of perishable substances and the deposit of the proceeds of the sale with the court. Upon a revocation order becoming final, all controlled substances may be forfeited to the state.
(d) The said state Board of Pharmacy or said appropriate department, board, or agency, as the case may be, shall promptly notify the bureau of all orders suspending or revoking registration and all forfeitures of controlled substances.
§60A-3-305. Order to show cause before denying, suspending, etc., registration; proceedings thereon; when order not required.
(a) Before denying, suspending, or revoking a registration, or refusing a renewal of registration, the said state Board of Pharmacy or said appropriate department, board, or agency, as the case may be, shall serve upon the applicant or registrant an order to show cause why registration should not be denied, suspended, or revoked, or why the renewal should not be refused. The order to show cause shall contain a statement of the basis therefor and shall call upon the applicant or registrant to appear before the said state Board of Pharmacy or said appropriate department, board, or agency, as the case may be, at a time and place not less than thirty days after the date of service of the order, but in the case of denial or renewal of registration the show cause order shall be served not later than thirty days before the expiration of the registration. These proceedings shall be conducted in accordance with article five, chapter twenty-nine-a of this code without regard to any criminal prosecution or other proceedings. Proceedings to refuse renewal of registration shall not abate the existing registration which shall remain in effect pending the outcome of the administrative hearing.
(b) The said state Board of Pharmacy or said appropriate department, board, or agency, as the case may be, may suspend, without an order to show cause, any registration simultaneously with the institution of proceedings under section 304, or where renewal of registration is refused, if it finds that there is an imminent danger to the public health or safety which warrants this action. The suspension shall continue in effect until the conclusion of the proceedings, including judicial review thereof unless sooner withdrawn by the said state Board of Pharmacy or said appropriate department, board, or agency, as the case may be, or dissolved by a court of competent jurisdiction.
§60A-3-306. Records of registrants.
Persons registered to manufacture, distribute, or dispense controlled substances under this act shall keep records and maintain inventories in conformance with the record-keeping and inventory requirements of federal law and with any additional rules the said state Board of Pharmacy or said appropriate department, board, or agency, as the case may be issued.
§60A-3-307. Order forms.
Controlled substances in Schedules I and II shall be distributed by a registrant to another registrant only pursuant to an order form. Compliance with the provisions of federal law respecting order forms shall be deemed compliance with this section.
§60A-3-308. Prescriptions.
(a) Except when dispensed directly by a practitioner, other than a pharmacy, to an ultimate user, no controlled substance in Schedule II may be dispensed without the lawful prescription of a practitioner.
(b) In emergency situations, as defined by the rule of the said appropriate department, board, or agency, Schedule II drugs may be dispensed upon the oral prescription of a practitioner, reduced promptly to writing, and filed by the pharmacy. Prescription shall be retained in conformity with the requirements of section three hundred six of this article. No prescription for a Schedule II substance may be refilled.
(c) Except when dispensed directly by a practitioner, other than a pharmacy, to an ultimate user, a controlled substance included in Schedule III or IV, which is a prescription drug as determined under appropriate state or federal statute, shall not be dispensed without a lawful prescription of a practitioner. The prescription shall not be filled or refilled more than six months after the date thereof or be refilled more than five times unless renewed by the practitioner.
(d) (1) A controlled substance included in Schedule V shall not be distributed or dispensed other than for a medicinal purpose: Provided, That buprenorphine shall be dispensed only by prescription pursuant to subsections (a), (b) and (c) of this section: Provided, however, That the controlled substances included in subsection (e), section two hundred twelve, article two of this chapter shall be dispensed, sold or distributed only by a physician, in a pharmacy by a pharmacist or pharmacy technician, or health care professional.
(2) If the substance described in subsection (e), section two hundred twelve, article two of this chapter is dispensed, sold, or distributed in a pharmacy:
(A) The substance shall be dispensed, sold, or distributed only by a pharmacist or a pharmacy technician; and
(B) Any person purchasing, receiving, or otherwise acquiring any such substance shall produce a photographic identification issued by a state or federal governmental entity reflecting his or her date of birth.
ARTICLE 4. OFFENSES AND PENALTIES. §60A-4-401. Prohibited acts A; penalties.
(a) Except as authorized by this act, it is unlawful for any person to manufacture, deliver, or possess with intent to manufacture or deliver a controlled substance.
Any person who violates this subsection with respect to:
(i) A controlled substance classified in Schedule I or II, which is a narcotic drug or which is methamphetamine, is guilty of a felony and, upon conviction thereof, may be imprisoned in a state correctional facility for not less than one year nor more than 15 years, or fined not more than $25,000, or both fined and imprisoned;
(ii) Any other controlled substance classified in Schedule I, II, or III is guilty of a felony and, upon conviction thereof, may be imprisoned in a state correctional facility for not less than one year nor more than five years, or fined not more than $15,000, or both fined and imprisoned;
(iii) A substance classified in Schedule IV is guilty of a felony and, upon conviction thereof, may be imprisoned in a state correctional facility for not less than one year nor more than three years, or fined not more than $10,000, or both fined and imprisoned;
(iv) A substance classified in Schedule V is guilty of a misdemeanor and, upon conviction thereof, may be confined in jail for not less than six months nor more than one year, or fined not more than $5,000, or both fined and confined: Provided, That for offenses relating to any substance classified as Schedule V in §60A-10-1 et seq. of this code, the penalties established in the said article apply.
(b) Except as authorized by this act, it is unlawful for any person to create, deliver, or possess with intent to deliver, a counterfeit substance.
Any person who violates this subsection with respect to:
(i) A counterfeit substance classified in Schedule I or II, which is a narcotic drug, or methamphetamine, is guilty of a felony and, upon conviction thereof, may be imprisoned in a state correctional facility for not less than one year nor more than 15 years, or fined not more than $25,000, or both fined and imprisoned;
(ii) Any other counterfeit substance classified in Schedule I, II, or III is guilty of a felony and, upon conviction thereof, may be imprisoned in a state correctional facility for not less than one year nor more than five years, or fined not more than $15,000, or both fined and imprisoned;
(iii) A counterfeit substance classified in Schedule IV is guilty of a felony and, upon conviction thereof, may be imprisoned in a state correctional facility for not less than one year nor more than three years, or fined not more than $10,000, or both fined and imprisoned;
(iv) A counterfeit substance classified in Schedule V is guilty of a misdemeanor and, upon conviction thereof, may be confined in jail for not less than six months nor more than one year, or fined not more than $5,000, or both fined and confined: Provided, That for offenses relating to any substance classified as Schedule V in §60A-10-1 et seq. of this code, the penalties established in the said article apply.
(c) It is unlawful for any person knowingly or intentionally to possess a controlled substance unless the substance was obtained directly from, or pursuant to, a valid prescription or order of a practitioner while acting in the course of his or her professional practice, or except as otherwise authorized by this act. Any person who violates this subsection is guilty of a misdemeanor, and disposition may be made under §60A-4-407 of this code, subject to the limitations specified in said section, or upon conviction thereof, the person may be confined in jail not less than 90 days nor more than six months, or fined not more than $1,000, or both fined and confined: Provided, That notwithstanding any other provision of this act to the contrary, any first offense for possession of synthetic cannabinoids as defined by §60A-1-101(d)(32) of this code; 3,4-methylenedioxypyrovalerone (MPVD) and 3,4-methylenedioxypyrovalerone and/or mephedrone as defined in §60A-1-101(f) of this code; or less than 15 grams of marijuana, shall be disposed of under §60A-4-407 of this code.
(d) It is unlawful for any person knowingly or intentionally:
(1) To create, distribute, deliver, or possess with intent to distribute or deliver, an imitation controlled substance; or
(2) To create, possess, sell, or otherwise transfer any equipment with the intent that the equipment shall be used to apply a trademark, trade name, or other identifying mark, imprint, number, or device, or any likeness thereof, upon a counterfeit substance, an imitation controlled substance, or the container or label of a counterfeit substance or an imitation controlled substance.
(3) Any person who violates this subsection is guilty of a misdemeanor and, upon conviction thereof, may be confined in jail for not less than six months nor more than one year, or fined not more than $5,000, or both fined and confined. Any person 18 years old or more who violates subdivision (1) of this subsection and distributes or delivers an imitation controlled substance to a minor child who is at least three years younger than that person is guilty of a felony and, upon conviction thereof, may be imprisoned in a state correctional facility for not less than one year nor more than three years, or fined not more than $10,000, or both fined and imprisoned.
(4) The provisions of subdivision (1) of this subsection shall not apply to a practitioner who administers or dispenses a placebo.
§60A-4-402. Prohibited acts B; penalties.
(a) It is unlawful for any person:
(1) Who is subject to article 3 to distribute or dispense a controlled substance in violation of section 308;
(2) Who is a registrant, to manufacture a controlled substance not authorized by his registration, or to distribute or dispense a controlled substance not authorized by his registration to another registrant or other authorized person;
(3) To refuse or fail to make, keep, or furnish any record, notification, order form, statement, invoice, or information required under this act;
(4) To refuse any entry into any premises for any inspection authorized by this act; or
(5) Knowingly to keep or maintain any store, shop, warehouse, dwelling, building, vehicle, boat, aircraft, or other structure or place, which is resorted to by persons using controlled substances in violation of this act for the purpose of using these substances, or which is used for keeping or selling them in violation of this act.
(b) Any person who violates this section is guilty of a misdemeanor, and, upon conviction, may be confined in the county jail for not less than six months nor more than one year, or fined not more than $25,000, or both.
(c) Notwithstanding any other provision of this act to the contrary, any first offense for distributing less than 15 grams of marihuana without any remuneration shall be disposed of under section 407.
§60A-4-403. Prohibited acts C; penalties.
(a) It is unlawful for any person knowingly or intentionally:
(1) To distribute as a registrant a controlled substance classified in Schedule I or II, except pursuant to an order form as required by section 307 of this act;
(2) To use in the course of the manufacture or distribution of a controlled substance a registration number which is fictitious, suspended, revoked, or issued to another person;
(3) To acquire or obtain possession of a controlled substance by misrepresentation, fraud, forgery, theft, deception, or subterfuge;
(4) To furnish false or fraudulent material information in, or omit any material information from, any application, report, or other document required to be kept or filed under this act, or any record required to be kept by this act; or
(5) To make, distribute, or possess any punch, die, plate, stone, or other thing designed to print, imprint, or reproduce the trademark, trade name, or other identifying mark, imprint, or device of another or any likeness of any of the foregoing upon any drug or container or labeling thereof so as to render the drug a counterfeit substance.
(b) Any person who violates this section is guilty of a felony and, upon conviction, may be imprisoned in a correctional facility for not less than one year nor more than four years, or fined not more than $30,000, or both.
§60A-4-403a. Prohibition of illegal drug paraphernalia businesses; definitions; places deemed common and public nuisances; abatement; suit to abate nuisances; injunction; search warrants; forfeiture of property; penalties.
(a) Any person who conducts, finances, manages, supervises, directs, or owns all or part of an illegal drug paraphernalia business is guilty of a misdemeanor, and, upon conviction thereof, shall be fined not more than $5,000, or confined in jail not less than six months nor more than one year, or both.
(b) A person violates subsection (a) of this section when:
(1) The person conducts, finances, manages, supervises, directs, or owns all or part of a business which for profit, in the regular course of business or as a continuing course of conduct, manufactures, sells, stores, possesses, gives away or furnishes objects designed to be primarily useful as drug devices.
(2) The person knows or has reason to know that the design of such objects renders them primarily useful as drug devices.
(c) As used in this section, "drug device" means an object usable for smoking marijuana, for smoking controlled substances defined as tetrahydrocannabinol, or for ingesting or inhaling cocaine, and includes, but is not limited to:
(i) Metal, wooden, acrylic, glass, stone, plastic, or ceramic pipes with or without screens, permanent screens, hashish heads, or punctured metal bowls;
(ii) Water pipes;
(iii) Carburetion tubes and devices;
(iv) Smoking and carburetion masks;
(v) Roach clips; meaning objects used to hold burning material, such as a marijuana cigarette, that has become too small or too short to be held in the hand;
(vi) Chamber pipes;
(vii) Carburetor pipes;
(viii) Electric pipes;
(ix) Air-driven pipes;
(x) Chillums;
(xi) Bongs;
(xii) Ice pipes or chillers; and
(xiii) Miniature cocaine spoons, and cocaine vials.
In any prosecution under this section, the question of whether an object is a drug-device shall be a question of fact.
(d) A place where drug devices are manufactured, sold, stored, possessed, given away, or furnished in violation of this section shall be deemed a common or public nuisance. Conveyances or vehicles of any kind shall be deemed places within the meaning of this section and maybe proceeded against under the provisions of subsection (e) of this section. A person who shall maintain, or shall aid or abet or knowingly be associated with others in maintaining such common or public nuisance shall be guilty of a misdemeanor, and, upon conviction thereof, shall be punished by a fine of not more than $1,000, or by confinement in jail not more than six months for each offense, and judgment shall be given that such nuisance is abated or closed as a place for the manufacture, sale, storage, possession, giving away or furnishing of drug devices.
(e) The prosecuting attorney or a citizen of the county or municipality where a nuisance as defined in subsection (d) is located, may maintain a suit in the name of the state to abate and perpetually enjoin the same. Circuit courts shall have jurisdiction thereof. The injunction may be granted at the commencement of the suit and no bond shall be required if such action for injunction be brought by the prosecuting attorney. If such a suit for injunction be brought or maintained by a citizen of the county or municipality where such a nuisance is alleged to be located, then the court may require a bond as in other cases of an injunction. On the finding that the material allegations of the complaint are true, the court or judge thereof in vacation shall order the injunction for such period of time as it or he may think proper, with the right to dissolve the injunction upon the application of the owner of the place, if a proper case is shown for such dissolution.
The continuance of the injunction as provided in this section may be ordered, although the place complained of may not at the time of hearing be unlawfully used.
(f) If there be complaint on oath or affirmation supported by affidavit or affidavits setting forth the facts for such belief that drug devices are being manufactured, sold, kept, stored or in any manner held, used or concealed in a particular house or another place with intent to engage in illegal drug paraphernalia business in violation of the law, a magistrate or a circuit court, or the judge thereof in the vacation to whom such complaint is made, if satisfied that there is probable cause for such belief, shall issue a warrant to search such house or another place for such devices. Such warrants, except as herein otherwise provided, shall be issued, directed, and executed in accordance with the laws of West Virginia pertaining to search warrants. Warrants issued under this section for the search of any automobile, boat, conveyance or vehicle, or for the search of any trunk, grip or another article of baggage, for such devices, may be executed in any part of the state where the same is overtaken and shall be made returnable before any magistrate or circuit court, or the judge thereof in vacation, within whose jurisdiction such automobile, boat, conveyance, vehicle, trunk, grip or another article of baggage, or any of them, were transported or attempted to be transported.
An officer charged with the execution of a warrant issued under this section, may, whenever it is necessary, break open and enter a house, or another place herein described.
(g) Any property, including money, used in violation of the provisions of this section may be seized and forfeited to the state.
§60A-4-404. Penalties under other laws.
Any penalty imposed for violation of this act is in addition to, and not in lieu of, any civil or administrative penalty or sanction otherwise authorized by law.
§60A-4-405. Bar to prosecution.
If a violation of this act is a violation of federal law or the law of another state, a conviction or acquittal under federal law or the law of another state for the same act is a bar to prosecution in this state.
§60A-4-406. Distribution to persons under the age of 18 by persons over the age of 21; distribution by persons 18 or over in, on, or within 1,000 feet of, school or college; distribution by persons 18 or over in, on, or within 200 feet of a public library; increasing mandatory period of incarceration prior to parole eligibility.
(a) Notwithstanding any other provision of law to the contrary, a person is ineligible for parole for a period of three years if he or she is sentenced to the custody of the Commissioner of Corrections and Rehabilitation, for service of a sentence of incarceration and is convicted of a felony violation under the provisions of §60A-4-401(a)(i) of this code for distribution of a controlled substance and:
(1) Is 21 years of age or older at the time of the distribution upon which the conviction is based, and the person to whom the controlled substance was distributed was under the age of 18 years at the time of the distribution;
(2) Is 18 years of age or older and the distribution upon which the conviction is based occurred in, on, or within 1,000 feet of, the real property comprising a public or private elementary, vocational or secondary school or a public or private college, junior college or university in this state; or
(3) Is 18 years of age or older and the distribution upon which the conviction is based occurred in, on, or within 200 feet of, the real property comprising a public library in this state.
(b) Notwithstanding any other provision of law to the contrary, a person is ineligible for parole for a period of two years if he or she is sentenced to the custody of the Commissioner of Corrections and Rehabilitation, for service of a sentence of incarceration and is convicted of a felony violation under the provisions of §60A-4-401(a)(ii) of this code for distribution of a controlled substance and:
(1) Is 21 years of age or older at the time of the distribution upon which the conviction is based, and the person to whom the controlled substance was distributed was under the age of 18 years at the time of the distribution;
(2) Is 18 years of age or older and the distribution upon which the conviction is based occurred in, on, or within 1,000 feet of, the real property comprising a public or private elementary, vocational or secondary school or a public or private college, junior college or university in this state; or
(3) Is 18 years of age or older and the distribution upon which the conviction is based occurred in, on, or within 200 feet of, the real property comprising a public library in this state.
(c) The existence of any fact which would make any person subject to the provisions of this section may not be considered unless the fact is clearly stated and included in the indictment or presentment by which the person is charged and is either:
(1) Found by the court upon a plea of guilty or nolo contendere;
(2) Found by the jury, if the matter be tried before a jury, upon submission to the jury of a special interrogatory for such purpose; or
(3) Found by the court, if the matter is tried by the court without a jury.
(d) Nothing in this section limits the sentencing alternatives made available to circuit court judges under other provisions of this code.
§60A-4-407. Conditional discharge for the first offense of possession.
(a) Whenever any person who has not previously been convicted of any offense under this chapter or under any statute of the United States or of any state relating to narcotic drugs, marihuana, or stimulant, depressant, or hallucinogenic drugs, pleads guilty to or is found guilty of possession of a controlled substance under section 401(c), the court, without entering a judgment of guilt and with the consent of the accused, may defer further proceedings and place him or her on probation upon terms and conditions. Upon violation of a term or condition, the court may enter an adjudication of guilt and proceed as otherwise provided. Upon fulfillment of the terms and conditions, the court shall discharge the person and dismiss the proceedings against him or her. Discharge and dismissal under this section shall be without adjudication of guilt and is not a conviction for purposes of this section or for purposes of disqualifications or disabilities imposed by law upon conviction of a crime, including the additional penalties imposed for second or subsequent convictions under section 408. The effect of the dismissal and discharge shall be to restore the person in contemplation of law to the status he or she occupied prior to arrest and trial. No person as to whom a dismissal and discharge have been effected shall be thereafter held to be guilty of perjury, false swearing, or otherwise giving a false statement by reason of his or her failure to disclose or acknowledge his or her arrest or trial in response to any inquiry made of him or her for any purpose. There may be only one discharge and dismissal under this section with respect to any person.
(b) After a period of not less than six months which shall begin to run immediately upon the expiration of a term of probation imposed upon any person under this chapter, the person may apply to the court for an order to expunge from all official records all recordations of his or her arrest, trial, and conviction, pursuant to this section. If the court determines after a hearing that the person during the period of his or her probation and during the period of time prior to his or her application to the court under this section has not been guilty of any serious or repeated violation of the conditions of his or her probation, it shall order the expungement.
(c) Notwithstanding any provision of this code to the contrary, any person prosecuted pursuant to the provisions of this article whose case is disposed of pursuant to the provisions of this section shall be liable for any court costs assessable against a person convicted of a violation of section 401(c) of this article. Payment of such costs may be made a condition of probation.
The costs assessed pursuant to this section, whether as a term of probation or not, shall be distributed as other court costs in accordance with section two, article three, chapter fifty, section four, article two-a, chapter fourteen, section four, article twenty-nine, chapter thirty and sections two, seven and ten, article five, chapter sixty-two of this code.
§60A-4-407a. Authorizing additional requirements to obtain a final order of discharge and dismissal for persons charged with possession of controlled substances.
(a) Notwithstanding any provision of this code to the contrary, when a person pleads guilty or is found guilty of a violation of §60A-4-401(c) of this code, or a municipal ordinance containing the same element where the controlled substance possessed is listed in §60A-2-204 of this code, other than marijuana, or is a controlled substance listed in §60A-2-206, §60A-2-208, or §60A-2-210 of this code, the court may, as an additional condition for the entry of a final order of discharge or dismissal under §60A-4-407 of this code or a municipal ordinance containing the same or substantially the same provision, require the defendant to be:
(1) Evaluated for admission into a drug court program; or
(2) Participate in a drug treatment program.
(b) If a defendant is determined to be an appropriate candidate for admission to drug court or a drug treatment program, the court may make successful completion of a drug court or a drug treatment program a requirement for obtaining a final order of discharge and dismissal.
§60A-4-408. Second or subsequent offenses.
(a) Any person convicted of a second or subsequent offense under this act may be imprisoned for a term up to twice the term otherwise authorized, fined an amount up to twice that otherwise authorized, or both. When a term of imprisonment is doubled under section 406, such term of imprisonment shall not be further increased for such offense under this subsection (a), even though such term of imprisonment is for a second or subsequent offense.
(b) For purposes of this section, an offense is considered a second or subsequent offense, if, prior to his conviction of the offense, the offender has at any time been convicted under this act or under any statute of the United States or of any state relating to narcotic drugs, marihuana, depressant, stimulant, or hallucinogenic drugs.
(c) This section does not apply to offenses under section 401(c).
§60A-4-409. Prohibited acts – Transportation of controlled substances into the state; penalties.
(a) Except as otherwise authorized by the provisions of this code, it is unlawful for any person to transport or cause to be transported into this state a controlled substance with the intent to deliver the same or with the intent to manufacture a controlled substance.
(b) Any person who violates this section with respect to:
(1) A controlled substance classified in Schedule I or II, which is a narcotic drug, shall be guilty of a felony and, upon conviction, may be imprisoned in the state correctional facility for not less than one year nor more than fifteen years, or fined not more than $25,000, or both;
(2) Any other controlled substance classified in Schedule I, II, or III shall be guilty of a felony and, upon conviction, may be imprisoned in the state correctional facility for not less than one year nor more than ten years, or fined not more than $15,000, or both: Provided, That for the substance marihuana, as scheduled in subdivision (24) subsection (d), section two hundred four, article two of this chapter, the penalty, upon conviction of a violation of this subsection, shall be that set forth in subdivision (3) of this subsection.
(3) A substance classified in Schedule IV shall be guilty of a felony and, upon conviction, may be imprisoned in the state correctional facility for not less than one year nor more than five years, or fined not more than $10,000, or both;
(4) A substance classified in Schedule V shall be guilty of a misdemeanor and, upon conviction, may be confined in jail for not less than six months nor more than one year, or fined not more than $5,000, or both: Provided, That for offenses relating to any substance classified as Schedule V in article ten of this chapter, the penalties established in the said article apply.
(c) Notwithstanding the provisions of subsection (b) of this section, any person violating or causing a violation of subsection (a) of this section involving one kilogram or more of heroin, five kilograms or more of cocaine or cocaine base, one hundred grams or more of phencyclidine, ten grams or more of lysergic acid diethylamide, or fifty grams or more of methamphetamine or five hundred grams of a substance or material containing a measurable amount of methamphetamine, is guilty of a felony and, upon conviction thereof, shall be imprisoned in a state correctional facility for a determinate sentence of not less than two nor more than thirty years.
(d) Notwithstanding the provisions of subsection (b) of this section, any person violating or causing a violation of subsection (a) of this section involving one hundred but fewer than 1000 grams of heroin, not less than five hundred but fewer than 5,000 grams of cocaine or cocaine base, not less than ten but fewer than ninety-nine grams of phencyclidine, not less than one but fewer than ten grams of lysergic acid diethylamide, or not less than five but fewer than fifty grams of methamphetamine or not less than fifty grams but fewer than five hundred grams of a substance or material containing a measurable amount of methamphetamine, is guilty of a felony and, upon conviction thereof, shall be imprisoned in a state correctional facility for a determinate sentence of not less than two nor more than twenty years.
(e) Notwithstanding the provisions of subsection (b) of this section, any person violating or attempting to violate the provisions of subsection (a) of this section involving not less than ten grams nor more than one hundred grams of heroin, not less than fifty grams nor more than five hundred grams of cocaine or cocaine base, not less than two grams nor more than ten grams of phencyclidine, not less than two hundred micrograms nor more than one gram of lysergic acid diethylamide, or not less than four hundred ninety-nine milligrams nor more than five grams of methamphetamine or not less than twenty grams nor more than fifty grams of a substance or material containing a measurable amount of methamphetamine is guilty of a felony and, upon conviction thereof, shall be imprisoned in a state correctional facility for a determinate sentence of not less than two nor more than fifteen years.
(f) The offense established by this section shall be in addition to and a separate and distinct offense from any other offense set forth in this code.
§60A-4-410. Prohibited acts -- Withholding information from practitioner; additional controlled substances; penalties.
(a) It is unlawful for a patient, in an attempt to obtain a prescription for a controlled substance, to knowingly withhold from a practitioner, that the patient has obtained a prescription for a controlled substance of the same or similar therapeutic use in a concurrent time period from another practitioner.
(b) Any person who violates this section is guilty of a misdemeanor and, upon conviction thereof, may be confined in jail for not more than nine months, or fined not more than $2,500, or both fined and confined.
(c) The offense established by this section is in addition to and a separate and distinct offense from any other offense set forth in this code.
§60A-4-411. Operating or attempting to operate clandestine drug laboratories; offenses; penalties.
(a) Any person who operates or attempts to operate a clandestine drug laboratory is guilty of a felony and, upon conviction, shall be confined in a state correctional facility for not less than two years nor more than ten years or fined not less than $5,000 nor more than $25,000, or both.
(b) Any person who operates or attempts to operate a clandestine drug laboratory and who as a result of, or in the course of doing so, causes to be burned any dwelling, outbuilding, building, or structure of any class or character is guilty of a felony and, upon conviction thereof, shall be fined not less than $1,000 nor more than $5,000, or imprisoned in a state correctional facility for not less than one nor more than five years, or both fined and imprisoned.
(c) For purposes of this section, a “clandestine drug laboratory” means any property, real or personal, on or in which a person assembles any chemicals or equipment or a combination thereof for the purpose of manufacturing methamphetamine, methylenedioxymethamphetamine, or lysergic acid diethylamide in violation of the provisions of section four hundred one of this article.
(d) The offenses in subsections (a) and (b) of this section are separate and distinct offenses and subsection (a) of this section shall not be construed to be a lesser included offense of subsection (b) of this section.
(e) For purposes of section one, article two of this chapter, both subsection (a) and (b) of this section shall be deemed qualifying felony offenses of manufacturing and delivery of a controlled substance.
(f) Any person convicted of a violation of subsection (a) or (b) of this section shall be responsible for all reasonable costs, if any, associated with remediation of the site of the clandestine drug laboratory.
§60A-4-412. Defeating drug and alcohol screening tests; penalties.
(a) Any person who:
(1) Knowingly sells, gives away, distributes, or markets any substance or product in this state or transports such a substance or product into this state with the intent that the substance or product will be used to defeat a drug or alcohol screening test;
(2) Attempts to defeat a drug or alcohol screening test by the substitution of a false sample;
(3) Knowingly advertises for sale or distribution any substance or product the advertised purpose of which is to defeat a bodily fluid screening test for drugs or alcohol;
(4) Adulterates a bodily fluid sample with the intent to defeat a drug or alcohol screening test;
(5) Knowingly possesses adulterants for the purpose of defeating a drug or alcohol screening test; or
(6) Knowingly sells adulterants which are intended to be used to adulterate urine or another bodily fluid sample for the purpose of defeating a drug or alcohol screening test.
(b) A person who violates a provision of subsection (a) of this section:
(1) For a first offense is guilty of a misdemeanor and, upon conviction, shall be fined not more than $1,000;
(2) For a second offense is guilty of a misdemeanor and, upon conviction, be fined not more than $5,000; and
(3) For a third or subsequent offense is guilty of a misdemeanor and, upon conviction, be fined not more than $10,000 or confined in the regional jail for not more than one year, or both.
(c) As used in this section, "adulterate" means a substance that is not expected to be in human fluids but that is a concentration so high that it is not consistent with human bodily fluids, including, but not limited to:
(1) Bleach;
(2) Chromium;
(3) Creatinine;
(4) Detergent;
(5) Glutaraldehyde;
(6) Glutaraldehyde/squalene;
(7) Hydrochloric acid;
(8) Hydroiodic acid;
(9) Iodine;
(10) Nitrite;
(11) Peroxidase;
(12) Potassium dichromate;
(13) Potassium nitrate;
(14) Pyridinium chlorochromate; and
(15) Sodium nitrite.
§60A-4-413. Unlawful production, manufacture, or possession of Salvia divinorum.
(a) For purposes of this section, "Salvia divinorum" means an herb belonging to the Lamiaceae family, a genus of Salvia, species of Divinorum, with common names including, but not limited to, "Salvia," "Ska Pastora," "Shepherdess's Herb," "Maria Pastora," "yerba de Maria," "Purple Sticky" and "Sally-D."
(b) It is unlawful for any person to knowingly or intentionally manufacture or possess an extract, compound, concentrate, or other processed substance intended for human consumption which contains Salvia divinorum, unless the substance was obtained directly from, or pursuant to, a valid prescription or order of a licensed physician or dispensed by a pharmacist for a recommended or medically necessary therapeutic use. Any person who violates this subsection is guilty of a misdemeanor, and disposition may be made under section four hundred seven of this article, subject to the limitations specified in said section, or upon conviction, such person may be confined in jail not more than six months, or fined not more than $1,000, or both. Notwithstanding any other provision of this code to the contrary, any first offense for possession of Salvia divinorum shall be disposed of under section four hundred seven of this article.
(c) The provisions of this section shall not apply to licensed physicians, pharmacists, and accredited hospitals and teaching facilities engaged in the research or study of Salvia divinorum, and shall not include any person participating in clinical trials involving the use of Salvia divinorum.
§60A-4-414. Conspiracy.
(a) Any person who willfully conspires with one or more persons to commit a felony violation of section four hundred one of this article, if one or more of such persons does any act to effect the object of the conspiracy, is guilty of a felony and, upon conviction thereof, shall be imprisoned in a state correctional facility for a determinate sentence of not less than two nor more than ten years: Provided, That the provisions of this subsection are inapplicable to felony violations of section four hundred one of this article prohibiting the manufacture, delivery or possession with intent to manufacture or deliver marijuana.
(b) Notwithstanding the provisions of subsection (a) of this section, any person who willfully conspires with one or more persons to manufacture, deliver or possess with intent to manufacture or deliver one kilogram or more of heroin, five kilograms or more of cocaine or cocaine base, one hundred grams or more of phencyclidine, ten grams or more of lysergic acid diethylamide, or fifty grams or more of methamphetamine or five hundred grams of a substance or material containing a measurable amount of methamphetamine, if one or more of such persons does any act to effect the object of the conspiracy, is guilty of a felony and, upon conviction thereof, shall be imprisoned in a state correctional facility for a determinate sentence of not less than two nor more than thirty years.
(c) Notwithstanding the provisions of subsection (a) of this section, any person who willfully conspires with one or more persons to manufacture, deliver or possess with intent to manufacture or deliver not less than one hundred but fewer than one thousand grams of heroin, not less than five hundred but fewer than five thousand grams of cocaine or cocaine base, not less than ten but fewer than one hundred grams of phencyclidine, not less than one but fewer than ten grams of lysergic acid diethylamide, or not less than five but fewer than fifty grams of methamphetamine or not less than fifty grams but fewer than five hundred grams of a substance or material containing a measurable amount of methamphetamine, if one or more of such persons does any act to effect the object of the conspiracy, is guilty of a felony and, upon conviction thereof, shall be imprisoned in a state correctional facility for a determinate sentence of not less than two nor more than twenty years.
(d) Notwithstanding the provisions of subsection (a) of this section, any person who willfully conspires with one or more persons to manufacture, deliver, possess with intent to manufacture or deliver not less than ten grams nor more than one hundred grams of heroin, not less than fifty grams nor more than five hundred grams of cocaine or cocaine base, not less than two grams nor more than ten grams of phencyclidine, not less than two hundred micrograms nor more than one gram of lysergic acid diethylamide, or not less than four hundred ninety-nine milligrams nor more than five grams of methamphetamine or not less than twenty grams nor more than fifty grams of a substance or material containing a measurable amount of methamphetamine, if one or more of such persons does any act to effect the object of the conspiracy, is guilty of a felony and, upon conviction thereof, shall be imprisoned in a state correctional facility for a determinate sentence of not less than two nor more than fifteen years.
(e) The trier of fact shall determine the quantity of the controlled substance attributable to the defendant beyond a reasonable doubt based on evidence adduced at trial.
(f) The determination of the trier of fact as to the quantity of controlled substance attributable to the defendant in a charge under this section may include all of the controlled substances manufactured, delivered or possessed with intent to deliver or manufacture by other participants or members of the conspiracy.
(g) Offenses in this section proscribing conduct involving lesser quantities are lesser included offenses of offenses proscribing conduct involving larger quantities.
(h) No person may be charged under the provisions of section thirty-one, article ten, chapter sixty-one of this code for conduct that is charged under this section.
(i) Nothing in this section may be construed to place any limitation whatsoever upon alternative sentencing options available to a court.
§60A-4-415. Unlawful manufacture, delivery, transport into the state, or possession of fentanyl.
(a) For purposes of this section,
(1) “Controlled substance” shall have the same meaning as provided in subsection (e), section one hundred one, article one of this chapter.
(2) “Fentanyl” refers to the substance identified in subdivision (9), subsection (c), section two hundred six, article two of this chapter, and any analog or derivative thereof.
(b) Any person who violates the provisions of subsection (a), section four hundred one of this article or section four hundred nine of this article in which fentanyl is a controlled substance involved in the offense, either alone or in combination with another controlled substance, shall be guilty of a felony, and upon conviction thereof, shall be punished in accordance with the following:
(1) If the net weight of fentanyl involved in the offense is less than one gram, such person shall be imprisoned in a correctional facility not less than two nor more than ten years.
(2) If the net weight of fentanyl involved in the offense is one gram or more but less than five grams, such person shall be imprisoned in a correctional facility not less than three nor more than fifteen years.
(3) If the net weight of fentanyl involved in the offense is five grams or more, such person shall be imprisoned in a correctional facility not less than four nor more than twenty years.
§60A-4-416. Drug delivery resulting in death; failure to render aid.
(a) Any person who knowingly and willfully delivers a controlled substance or counterfeit controlled substance in violation of the provisions of section four hundred one, article four of this chapter for an illicit purpose and the use, ingestion or consumption of the controlled substance or counterfeit controlled substance alone or in combination with one or more other controlled substances, proximately causes the death of a person using, ingesting or consuming the controlled substance, is guilty of a felony and, upon conviction thereof, shall be imprisoned in a state correctional facility for a determinate sentence of not less than three nor more than fifteen years.
(b) Any person who, while engaged in the illegal use of a controlled substance with another, who knowingly fails to seek medical assistance for such other person when the other person suffers an overdose of the controlled substance or suffers a significant adverse physical reaction to the controlled substance and the overdose or adverse physical reaction proximately causes the death of the other person, is guilty of a felony and, upon conviction thereof, shall be imprisoned for not less than one year nor more than five years.
§60A-4-417. Sale of dextromethorphan.
(a) As used in this section, "finished drug product" means a drug legally marketed under the Federal Food, Drug, and Cosmetic Act (21 U.S.C. § 321 et seq.) that is in the finished dosage form.
(b) A person may not knowingly or willfully sell or trade a finished drug product containing any quantity of dextromethorphan to a person under 18 years of age.
(c) A person under 18 years of age, unless an emancipated minor, may not purchase a finished drug product containing any quantity of dextromethorphan.
(d) A person making a retail sale of a finished drug product containing any quantity of dextromethorphan shall require and obtain proof of age from the purchaser before completing the sale, unless from the purchaser’s outward appearance the person making the sale would reasonably presume the purchaser to be at least 25 years of age.
(e) This section does not apply to a medication containing dextromethorphan that is sold pursuant to a valid prescription.
(f) Any person violating the provisions of this section is guilty of a misdemeanor and shall be fined not less than $100 nor more than $250.
ARTICLE 5. ENFORCEMENT AND ADMINISTRATIVE PROVISIONS.
§60A-5-501. Powers of enforcement personnel.
(a) Any member of the State Police, any sheriff, any deputy sheriff, any municipal police officer, and any campus police officer may in the enforcement of the provisions of this act:
(1) Carry firearms;
(2) Execute and serve search warrants, arrest warrants, subpoenas, and summonses issued under the authority of this state;
(3) Make arrests without warrant for any offense under this act committed in his presence, or if he has probable cause to believe that the person to be arrested has committed or is committing a violation of this act which may constitute a felony;
(4) Make seizures of property pursuant to this act; or
(5) Perform such other law-enforcement duties as said state Board of Pharmacy or said appropriate department, board, or agency, as specified in section 301, designates.
(b) All officers, agents, inspectors, and representatives of the said state Board of Pharmacy and of the said appropriate department, board, or agency, as specified in section 301, and members of the State Police may execute and serve administrative warrants issued incident to the enforcement of the provisions of this act. Any such officer, agent, inspector, and representative of the said state Board of Pharmacy and of the said appropriate department, board, or agency, as specified in said section 301, may:
(1) Execute and serve subpoenas and summonses issued under the authority of this state;
(2) Make arrests without warrant for any offense under this act committed in his presence, or if he has probable cause to believe that the person to be arrested has committed or is committing a violation of this act which may constitute a felony; or
(3) Make seizures of property pursuant to this act.
(c) All prosecuting attorneys and the Attorney General, or any of their assistants, shall assist in the enforcement of all provisions of this act and shall cooperate with all agencies charged with the enforcement of the laws of the United States, of this state, and of all other states relating to controlled substances.
§60A-5-502. Administrative inspections and warrants.
(a) Issuance and execution of administrative inspection warrants shall be as follows:
(1) A judge of any court of record in this state having criminal jurisdiction, and upon proper oath or affirmation showing probable cause, may issue warrants for the purpose of conducting administrative inspections authorized by this act or rules hereunder, and seizures of property appropriate to the inspections. For purposes of the issuance of administrative inspection warrants, probable cause exists upon showing a valid public interest in the effective enforcement of this act or rules hereunder, sufficient to justify administrative inspection of the area, premises, building, or conveyance in the circumstances specified in the application for the warrant;
(2) A warrant shall issue only upon an affidavit of a designated officer or employee having knowledge of the facts alleged, sworn to before the judge, and establishing the grounds for issuing the warrant. If the judge is satisfied that grounds for the application exist or that there is probable cause to believe they exist, he shall issue a warrant identifying the area, premises, building, or conveyance to be inspected, the purpose of the inspection, and, if appropriate, the type of property to be inspected, if any. The warrant shall:
(i) State the grounds for its issuance and the name of each person whose affidavit has been taken in support thereof;
(ii) Be directed to a person authorized by section 501 to execute it;
(iii) Command the person to whom it is directed to inspect the area, premises, building, or conveyance identified for the purpose specified and, if appropriate, direct the seizure of the property specified;
(iv) Identify the item or types of property to be seized, if any;
(v) Direct that it be served during normal business hours and designate the judge to whom it shall be returned.
(3) A warrant issued pursuant to this section must be executed and returned within ten days of its date unless, upon a showing of a need for additional time, the court orders otherwise. If property is seized pursuant to a warrant, a copy shall be given to the person from whom or from whose premises the property is taken, together with a receipt for the property taken. The return of the warrant shall be made promptly, accompanied by a written inventory of any property taken. The inventory shall be made in the presence of the person executing the warrant and of the person from whose possession or premises the property was taken, if present, or in the presence of at least one credible person other than the person executing the warrant. A copy of the inventory shall be delivered to the person from whom or from whose premises the property was taken and to the applicant for the warrant;
(4) The judge who has issued a warrant shall attach thereto a copy of the return and all papers returnable in connection therewith and file them with the clerk of the court.
(b) Administrative inspections of controlled premises shall be made in accordance with the following provisions:
(1) For purposes of this section only, "controlled premises" means:
(i) Places, where persons registered or exempted from registration requirements under this act, are required to keep records; and
(ii) Places including factories, warehouses, establishments, and conveyances in which persons registered or exempted from registration requirements under this act are permitted to hold, manufacture, compound, process, sell, deliver, or otherwise dispose of any controlled substance.
(2) When authorized by an administrative inspection warrant issued pursuant to subsection (a), any person authorized in subsection (b), section 501 of this article to execute and serve the same, upon presenting the warrant and appropriate credentials to the owner, operator, or agent in charge, may enter controlled premises for the purpose of conducting an administrative inspection.
(3) When authorized by an administrative inspection warrant, any such person may:
(i) Inspect and copy records required by this act to be kept;
(ii) Inspect, within reasonable limits and in a reasonable manner, controlled premises and all pertinent equipment finished and unfinished material, containers, and labeling found therein, and, except as provided in subsection (b) (5), all other things therein, including records, files, papers, processes, controls, and facilities bearing on violation of this act; and
(iii) Inventory any stock of any controlled substance therein and obtain samples thereof.
(4) This section does not prevent the inspection without a warrant of books and records pursuant to an administrative subpoena issued in accordance with any pertinent provision of this code, nor does it prevent entries and administrative inspections, including seizures of property, without a warrant:
(i) If the owner, operator, or agent in charge of the controlled premises consents;
(ii) In situations presenting imminent danger to health or safety;
(iii) In situations involving inspection of conveyances if there is reasonable cause to believe that the mobility of the conveyance makes it impracticable to obtain a warrant;
(iv) In any other exceptional or emergency circumstance where time or opportunity to apply for a warrant is lacking; or,
(v) In all other situations in which a warrant is not Constitutionally required.
(5) An inspection authorized by this section shall not extend to financial data, sales data, other than shipment data, or pricing data unless the owner, operator, or agent in charge of the controlled premises consents in writing.
§60A-5-503. Injunctions.
(a) The courts of record of this state have and may exercise jurisdiction to restrain or enjoin violations of this act.
(b) The defendant may demand trial by jury for an alleged violation of an injunction or restraining order under this section.
§60A-5-504. Cooperative arrangements; confidentiality; treatment of minor without knowledge or consent of parent or guardian.
(a) The State Board of Pharmacy and the appropriate departments, boards, and agencies, as specified in section 301, shall cooperate with federal and other state agencies in discharging their responsibilities concerning traffic in controlled substances and in suppressing the abuse of controlled substances. To this end, they may:
(1) Arrange for the exchange of information among government officials concerning the use and abuse of controlled substances;
(2) Coordinate and cooperate in training programs concerning controlled substance law enforcement at local and state levels;
(3) Cooperate with the bureau by establishing a centralized unit to accept, catalog, file, and collect statistics, including records of drug-dependent persons and other controlled substance law offenders within the state, and make the information available for federal, state, and local law enforcement purposes. They shall not furnish the name or identity of a patient or research subject whose identity could not be obtained under subsection (c); and
(4) Conduct programs of eradication aimed at destroying wild or illicit growth of plant species from which controlled substances may be extracted.
(b) Results, information, and evidence received from the bureau relating to the regulatory functions of this chapter, including results of inspections conducted by it may be relied on and acted upon by the state Board of Pharmacy in the exercise of its regulatory functions under this chapter.
(c) A practitioner engaged in medical practice or research is not required or compelled to furnish the name or identity of a patient or research subject to the state Board of Pharmacy or to the appropriate department, board, or agency by which he is licensed or registered, as specified in section 301, nor may he be compelled in any state or local civil, criminal, administrative, legislative, or other proceedings to furnish the name or identity of an individual that the practitioner is obligated to keep confidential.
(d) No mental health organization or hospital shall be compelled in any state or local civil, criminal, administrative, legislative, or another proceeding to furnish the name or identity of any person voluntarily requesting treatment for or rehabilitation from addiction to or dependency upon the use of a controlled substance as defined in article one of this chapter.
(e) Notwithstanding any other provision of law, any licensed physician or competent medically trained person under his direction may examine, diagnose, and treat any minor at his or her request for any addiction to or dependency upon the use of a controlled substance as defined in article one of this chapter without the knowledge or consent of the minor's parent or guardian. Such a physician and such other persons shall not incur any civil or criminal liability in connection therewith except for negligence or willful injury.
§60A-5-505.
Repealed
Acts, 1988 Reg. Sess., Ch. 23.
§60A-5-506. Burden of proof; liability of officers.
(a) It is not necessary for the state to negate any exemption or exception in this act in any complaint, information, indictment, or other pleading or in any trial, hearing, or other proceedings under this act. The burden of proof of any exemption or exception is upon the person claiming it.
(b) In the absence of proof that a person is the duly authorized holder of an appropriate registration or order form issued under this act, he is presumed not to be the holder of the registration or form. The burden of proof is upon him to rebut the presumption.
(c) No liability is imposed by this act upon any authorized state, county, or municipal officer, engaged in the lawful performance of his duties.
§60A-5-507. Judicial review.
All final determinations, findings, and conclusions of the said state Board of Pharmacy or the appropriate department, board, or agency, as specified in section 301, made under this act after hearing are final and conclusive decisions of the matters involved. Any person aggrieved by the decision may obtain a review of the decision pursuant to the provisions of articles five and six, chapter twenty-nine-a of this code.
§60A-5-508. Education and research.
(a) The said state Board of Pharmacy and the appropriate departments, boards, and agencies, as specified in section 301, and the division on alcoholism and drug abuse in the department of mental health (all hereinafter in this section referred to as "such agencies"), shall carry out educational programs designed to prevent and deter misuse and abuse of controlled substances. In connection with these programs they may:
(1) Promote better recognition of the problems of misuse and abuse of controlled substances within the regulated industry and among interested groups and organizations;
(2) Assist the regulated industry and interested groups and organizations in contributing to the reduction of misuse and abuse of controlled substances;
(3) Consult with interested groups and organizations to aid them in solving administrative and organizational problems;
(4) Evaluate procedures, projects, techniques, and controls conducted or proposed as part of educational programs on misuse and abuse of controlled substances;
(5) Disseminate the results of research on misuse and abuse of controlled substances to promote a better public understanding of what problems exist and what can be done to combat them; and
(6) Assist in the education and training of state and local law-enforcement officials in their efforts to control misuse and abuse of controlled substances.
(b) Such agencies shall encourage research on misuse and abuse of controlled substances. In connection with the research, and in furtherance of the enforcement of this act, such agencies may:
(1) Establish methods to assess accurately the effects of controlled substances and identify and characterize those with potential for abuse;
(2) Makes studies and undertake programs of research to:
(i) Develop new or improved approaches, techniques, systems, equipment, and devices to strengthen the enforcement of this act;
(ii) Determine patterns of misuse and abuse of controlled substances and the social effects thereof; and,
(iii) Improve methods for preventing, predicting, understanding, and dealing with the misuse and abuse of controlled substances; and,
(3) Enter into contracts with public agencies, institutions of higher education, and private organizations or individuals for the purpose of conducting research, demonstrations, or special projects which bear directly on misuse and abuse of controlled substances.
(c) Such agencies may enter into contracts for educational and research activities without performance bonds.
(d) Such agencies may authorize persons engaged in research on the use and effects of controlled substances to withhold the names and other identifying characteristics of individuals who are the subjects of the research. Persons who obtain this authorization are not compelled in any civil, criminal, administrative, legislative, or other proceedings to identify the individuals who are the subjects of research for which the authorization was obtained.
(e) Such agencies may authorize the possession and distribution of controlled substances by persons engaged in research. Persons who obtain this authorization are exempt from state prosecution for possession and distribution of controlled substances to the extent of the authorization.
§60A-5-509. Unlawful retaliation against health care providers.
(a) A health care provider has the right to exercise his or her professional judgment to decline to administer, dispense, or prescribe narcotics without being subject to actual or threatened acts of reprisal.
(b) It shall be unlawful for any person or entity to engage in any form of threats or reprisal, or to engage in, or hire, or conspire with, others to commit acts or activities of any nature, the purpose of which is to punish, embarrass, deny, or reduce privileges or compensation, or cause economic loss or to aid, abet, incite, compel, or coerce any person to engage in such threats or reprisal, against a health care provider as a result of, or in retaliation for, the refusal of that health care provider to administer, dispense, or prescribe narcotics.
(c) Any person or entity who violates the foregoing shall be subject to a private right of action by the affected health care provider and shall be liable in the amount of three times the economic loss sustained as a direct and proximate result of the reprisal.
(d) A health care provider that prevails in an action brought pursuant to this section shall be entitled to an award of costs and attorney fees.
ARTICLE 6. MISCELLANEOUS PROVISIONS.
§60A-6-601. Pending proceedings.
(a) The provisions of this act shall govern and control as to any offenses committed in violation thereof on and after the effective date of this act, and the provisions of articles eight, eight-a and eight-b, chapter sixteen of this code shall govern and control as to any offenses committed in violation of said articles, or any of them, prior to the effective date of this act, with like effect as to such prior offenses as if said articles had not been repealed and this act had not been enacted: Provided, That if the offense being prosecuted is similar to one set out in article four of this act, then the penalties under article four apply if they are less than those under prior law.
(b) Civil seizures of forfeitures and injunctive proceedings commenced prior to the effective date of this act are not affected by this act.
(c) All administrative proceedings pending under prior laws which are superseded by this act shall be continued and brought to a final determination in accord with the laws and rules in effect prior to the effective date of the act. Any substance controlled under prior law which is not listed within Schedules I through V, is automatically controlled without further proceedings and shall be listed in the appropriate schedule.
(d) The State Board of Pharmacy or the appropriate departments, boards, and agencies, as specified in section 301, shall initially permit persons to register who own or operate any establishment engaged in the manufacture, distribution, or dispensing of any controlled substance prior to the effective date of this act and who are registered or licensed by the state.
(e) This act applies to violations of law, seizures, and forfeiture, injunctive proceedings, administrative proceedings, and investigations that occur following its effective date.
§60A-6-602. Continuation of orders and rules.
Any orders and rules promulgated under any law affected by this act and in effect on the effective date of this act and not in conflict with it continue in effect until modified, superseded, or repealed.
§60A-6-603. Uniformity of interpretation.
This act shall be so applied and construed as to effectuate its general purpose to make uniform the law with respect to the subject of this act among those states which enact it.
§60A-6-604. Short title.
This act may be cited as the Uniform Controlled Substances Act.
§60A-6-605. Severability.
If any provision of this act or the application thereof to any person or circumstance is held invalid, such invalidity shall not affect other provisions or applications of the act, and to this end, the provisions of this act are hereby declared to be severable.
ARTICLE 9. CONTROLLED SUBSTANCES MONITORING. §60A-9-1. Short title.
This article shall be referred to as the West Virginia Controlled Substances Monitoring Act.
§60A-9-2. Establishment of program; purpose.
There is hereby established a West Virginia controlled substances monitoring act the purpose of which is to require the recordation and retention in a single repository of information regarding the prescribing, dispensing, and consumption of certain controlled substances.
§60A-9-3. Reporting system requirements; implementation; central repository requirement.
(a) The Board of Pharmacy shall implement a program wherein a central repository is established and maintained which shall contain such information as is required by the provisions of this article regarding Schedule II, III, and IV controlled substance prescriptions written or filled in this state. In implementing this program, the Board of Pharmacy shall consult with the West Virginia State Police, the licensing boards of practitioners affected by this article, and affected practitioners.
(b) The program authorized by subsection (a) of this section shall be designed to minimize inconvenience to patients, prescribing practitioners, and pharmacists while effectuating the collection and storage of the required information. The board shall allow reporting of the required information by electronic data transfer where feasible, and where not feasible, on reporting forms promulgated by the board. The information required to be submitted by the provisions of this article shall be required to be filed no more frequently than within twenty-four hours.
(c) (1) The board shall provide for the electronic transmission of the information required to be provided by this article by and through the use of a toll-free telephone line.
(2) A dispenser, who does not have an automated record-keeping system capable of producing an electronic report in the established format may request a waiver from electronic reporting. The request for a waiver shall be made to the board in writing and shall be granted if the dispenser agrees in writing to report the data by submitting a completed "Pharmacy Universal Claim Form" as defined by legislative rule.
§60A-9-4. Required information.
(a) The following individuals shall report the required information to the Controlled Substances Monitoring Program Database when:
(1) A medical services provider dispenses a controlled substance listed in Schedule II, III, IV, or V;
(2) A prescription for the controlled substance or opioid antagonist is filled by:
(A) A pharmacist or pharmacy in this state;
(B) A hospital, or other health care facility, for outpatient use; or
(C) A pharmacy or pharmacist licensed by the Board of Pharmacy, but situated outside this state for delivery to a person residing in this state; and
(3) A pharmacist or pharmacy sells an opioid antagonist.
(b) The above individuals shall in a manner prescribed by rules promulgated by the Board of Pharmacy pursuant to this article, report the following information, as applicable:
(1) The name, address, pharmacy prescription number, and Drug Enforcement Administration controlled substance registration number of the dispensing pharmacy or the dispensing physician or dentist;
(2) The full legal name, address, and birth date of the person for whom the prescription is written;
(3) The name, address, and Drug Enforcement Administration controlled substances registration number of the practitioner writing the prescription;
(4) The name and national drug code number of the Schedule II, III, IV, and V controlled substance or opioid antagonist dispensed;
(5) The quantity and dosage of the Schedule II, III, IV, and V controlled substance or opioid antagonist dispensed;
(6) The date the prescription was written and the date filled;
(7) The number of refills, if any, authorized by the prescription;
(8) If the prescription being dispensed is being picked up by someone other than the patient on behalf of the patient, information about the person picking up the prescription as set forth on the person’s government-issued photo identification card shall be retained in either print or electronic form until such time as otherwise directed by rule promulgated by the Board of Pharmacy; and
(9) The source of payment for the controlled substance dispensed.
(c) Whenever a medical services provider treats a patient for an overdose that has occurred as a result of illicit or prescribed medication, the medical service provider shall report the full legal name, address, and birth date of the person who is being treated, including any known ancillary evidence of the overdose. The Board of Pharmacy shall coordinate with the Division of Justice and Community Services and the Office of Drug Control Policy regarding the collection of overdose data.
(d) The Board of Pharmacy may prescribe by rule promulgated pursuant to this article the form to be used in prescribing a Schedule II, III, IV, and V substance or opioid antagonist if, in the determination of the Board of Pharmacy, the administration of the requirements of this section would be facilitated.
(e) Products regulated by the provisions of §60A-10-1 et seq. of this code shall be subject to reporting pursuant to the provisions of this article to the extent set forth in said article.
(f) Reporting required by this section is not required for a drug administered directly to a patient by a practitioner. Reporting is, however, required by this section for a drug dispensed to a patient by a practitioner. The quantity dispensed by a prescribing practitioner to his or her own patient may not exceed an amount adequate to treat the patient for a maximum of 72 hours with no greater than two 72-hour cycles dispensed in any 15-day period of time.
(g) The Board of Pharmacy shall notify a physician prescribing buprenorphine or buprenorphine/naloxone within 60 days of the availability of an abuse-deterrent or a practitioner-administered form of buprenorphine or buprenorphine/naloxone if approved by the Food and Drug Administration as provided in FDA Guidance to Industry. Upon receipt of the notice, a physician may switch his or her patients using buprenorphine or buprenorphine/naloxone to the abuse-deterrent or a practitioner-administered form of the drug.
§60A-9-4a. Verification of identity.
Prior to releasing a Schedule II, III, or IV controlled substance sold at retail, a pharmacist or pharmacy shall verify the full legal name, address, and birth date of the person picking up the controlled substance dispensed by requiring the presentation of a valid government-issued photo identification card. This information shall be reported in accordance with the provisions of this article.
§60A-9-5. Confidentiality; limited access to records; period of retention; no civil liability for required reporting.
(a)(1) The information required by this article to be kept by the Board of Pharmacy is confidential and not subject to the provisions of §29B-1-1 et seq. of this code or obtainable as discovery in civil matters absent a court order and is open to inspection only by inspectors and agents of the Board of Pharmacy, members of the West Virginia State Police expressly authorized by the Superintendent of the West Virginia State Police to have access to the information, authorized agents of local law-enforcement agencies as members of a federally affiliated drug task force, authorized agents of the federal Drug Enforcement Administration, duly authorized agents of the Bureau for Medical Services, duly authorized agents of the Office of the Chief Medical Examiner for use in post-mortem examinations, duly authorized agents of the Office of Health Facility Licensure and Certification for use in certification, licensure, and regulation of health facilities, duly authorized agents of licensing boards of practitioners in this state and other states authorized to prescribe Schedules II, III, and IV controlled substances, prescribing practitioners and pharmacists, a dean of any medical school or his or her designee located in this state to access prescriber level data to monitor prescribing practices of faculty members, prescribers, and residents enrolled in a degree program at the school where he or she serves as dean, a physician reviewer designated by an employer of medical providers to monitor prescriber level information of prescribing practices of physicians, advance practice registered nurses, or physician assistants in their employ, and a chief medical officer of a hospital or a physician designated by the chief executive officer of a hospital who does not have a chief medical officer, for prescribers who have admitting privileges to the hospital or prescriber level information, and persons with an enforceable court order or regulatory agency administrative subpoena. All law-enforcement personnel who have access to the Controlled Substances Monitoring Program Database shall be granted access in accordance with applicable state laws and the Board of Pharmacy’s rules, shall be certified as a West Virginia law-enforcement officer and shall have successfully completed training approved by the Board of Pharmacy. All information released by the Board of Pharmacy must be related to a specific patient or a specific individual or entity under investigation by any of the above parties except that practitioners who prescribe or dispense controlled substances may request specific data related to their Drug Enforcement Administration controlled substance registration number or for the purpose of providing treatment to a patient: Provided, That the West Virginia Controlled Substances Monitoring Program Database Review Committee established in §30A-9-5(b) of this code is authorized to query the database to comply with §30A-9-5(b) of this code.
(2) Subject to the provisions of §60A-9-5(a)(1) of this code, the Board of Pharmacy shall also review the West Virginia Controlled Substances Monitoring Program Database and issue reports that identify abnormal or unusual practices of patients and practitioners with prescriptive authority who exceed parameters as determined by the advisory committee established in this section. The Board of Pharmacy shall communicate with practitioners and dispensers to more effectively manage the medications of their patients in the manner recommended by the advisory committee. All other reports produced by the Board of Pharmacy shall be kept confidential. The Board of Pharmacy shall maintain the information required by this article for a period of not less than five years. Notwithstanding any other provisions of this code to the contrary, data obtained under the provisions of this article may be used for the compilation of educational, scholarly, or statistical purposes, and may be shared with the West Virginia Department of Health and Human Resources for those purposes, as long as the identities of persons or entities and any personally identifiable information, including protected health information, contained therein shall be redacted, scrubbed, or otherwise irreversibly destroyed in a manner that will preserve the confidential nature of the information. No individual or entity required to report under §60A-9-4 of this code may be subject to a claim for civil damages or other civil relief for the reporting of information to the Board of Pharmacy as required under and in accordance with the provisions of this article.
(3) The Board of Pharmacy shall establish an advisory committee to develop, implement, and recommend parameters to be used in identifying abnormal or unusual usage patterns of patients and practitioners with prescriptive authority in this state. This advisory committee shall:
(A) Consist of the following members: A physician licensed by the West Virginia Board of Medicine; a dentist licensed by the West Virginia Board of Dental Examiners; a physician licensed by the West Virginia Board of Osteopathic Medicine; a licensed physician certified by the American Board of Pain Medicine; a licensed physician board certified in medical oncology recommended by the West Virginia State Medical Association; a licensed physician board-certified in palliative care recommended by the West Virginia Center on End of Life Care; a pharmacist licensed by the West Virginia Board of Pharmacy; a licensed physician member of the West Virginia Academy of Family Physicians; an expert in drug diversion; and such other members as determined by the Board of Pharmacy.
(B) Recommend parameters to identify abnormal or unusual usage patterns of controlled substances for patients in order to prepare reports as requested in accordance with §60A-9-5(a)(2) of this code.
(C) Make recommendations for training, research, and other areas that are determined by the committee to have the potential to reduce inappropriate use of prescription drugs in this state, including, but not limited to, studying issues related to diversion of controlled substances used for the management of opioid addiction.
(D) Monitor the ability of medical services providers, health care facilities, pharmacists, and pharmacies to meet the 24-hour reporting requirement for the Controlled Substances Monitoring Program set forth in §60A-9-3 of this code, and report on the feasibility of requiring real-time reporting.
(E) Establish outreach programs with local law enforcement to provide education to local law enforcement on the requirements and use of the Controlled Substances Monitoring Program Database established in this article.
(b) The Board of Pharmacy shall create a West Virginia Controlled Substances Monitoring Program Database Review Committee of individuals consisting of two prosecuting attorneys from West Virginia counties, two physicians with specialties which require extensive use of controlled substances, and a pharmacist who is trained in the use and abuse of controlled substances. The review committee may determine that an additional physician who is an expert in the field under investigation be added to the team when the facts of a case indicate that the additional expertise is required. The review committee, working independently, may query the database based on parameters established by the advisory committee. The review committee may make determinations on a case-by-case basis on specific unusual prescribing or dispensing patterns indicated by outliers in the system or abnormal or unusual usage patterns of controlled substances by patients which the review committee has reasonable cause to believe necessitates further action by law enforcement or the licensing board having jurisdiction over the practitioners or dispensers under consideration. The licensing board having jurisdiction over the practitioner or dispenser under consideration shall report back to the Board of Pharmacy regarding any findings, investigation, or discipline resulting from the findings of the review committee within 30 days of resolution of any action taken by the licensing board resulting from the information provided by the Board of Pharmacy. The review committee shall also review notices provided by the chief medical examiner pursuant to §61-12-10(h) of this code and determine on a case-by-case basis whether a practitioner who prescribed or dispensed a controlled substance resulting in or contributing to the drug overdose may have breached professional or occupational standards or committed a criminal act when prescribing the controlled substance at issue to the decedent. Only in those cases in which there is reasonable cause to believe a breach of professional or occupational standards or a criminal act may have occurred, the review committee shall notify the appropriate professional licensing agency having jurisdiction over the applicable practitioner or dispenser and appropriate law-enforcement agencies and provide pertinent information from the database for their consideration. The number of cases identified shall be determined by the review committee based on a number that can be adequately reviewed by the review committee. The information obtained and developed may not be shared except as provided in this article and is not subject to the provisions of §29B-1-1 et seq. of this code or obtainable as discovering in civil matters absent a court order.
(c) The Board of Pharmacy is responsible for establishing and providing administrative support for the advisory committee and the West Virginia Controlled Substances Monitoring Program Database Review Committee. The advisory committee and the review committee shall elect a chair by majority vote. Members of the advisory committee and the review committee may not be compensated in their capacity as members but shall be reimbursed for reasonable expenses incurred in the performance of their duties.
(d) The Board of Pharmacy shall promulgate rules with advice and consent of the advisory committee, after consultation with the licensing boards set forth in §60A-9-5(d)(4) of this code and in accordance with the provisions of §29A-3-1 et seq. of this code. The Legislature finds that the changes made to this section during the course of the 2018 regular session of the Legislature constitute an emergency and the Board of Pharmacy shall promulgate emergency rules pursuant to the provisions of §29A-3-15 of this code to incorporate these modifications. The legislative rules must include, but shall not be limited to, the following matters:
(1) Identifying parameters used in identifying abnormal or unusual prescribing or dispensing patterns;
(2) Processing parameters and developing reports of abnormal or unusual prescribing or dispensing patterns for patients, practitioners, and dispensers;
(3) Establishing the information to be contained in reports and the process by which the reports will be generated and disseminated;
(4) Dissemination of these reports at least quarterly to:
(A) The West Virginia Board of Medicine codified in §30-3-1 et seq. of this code;
(B) The West Virginia Board of Osteopathic Medicine codified in §30-14-1 et seq. of this code;
(C) The West Virginia Board of Examiners for Registered Professional Nurses codified in §30-7-1 et seq. of this code;
(D) The West Virginia Board of Dentistry codified in §30-4-1 et seq. of this code;
(E) The West Virginia Board of Optometry codified in §30-8-1 et seq. of this code; and
(F) The West Virginia Board of Veterinary Medicine codified in §30-10-1 et seq. of this code; and
(5) Setting up processes and procedures to ensure that the privacy, confidentiality, and security of information collected, recorded, transmitted, and maintained by the review committee is not disclosed except as provided in this section.
(e) Persons or entities with access to the West Virginia Controlled Substances Monitoring Program Database pursuant to this section may, pursuant to rules promulgated by the Board of Pharmacy, delegate appropriate personnel to have access to said database.
(f) Good faith reliance by a practitioner on information contained in West Virginia Controlled Substances Monitoring Program Database in prescribing or dispensing or refusing or declining to prescribe or dispense a Schedule II, III, or IV controlled substance shall constitute an absolute defense in any civil or criminal action brought due to prescribing or dispensing or refusing or declining to prescribe or dispense.
(g) A prescribing or dispensing practitioner may notify law enforcement of a patient who, in the prescribing or dispensing practitioner’s judgment, may be in violation of §60A-4-410 of this code, based on information obtained and reviewed from the Controlled Substances Monitoring Program Database. A prescribing or dispensing practitioner who makes a notification pursuant to this subsection is immune from any civil, administrative, or criminal liability that otherwise might be incurred or imposed because of the notification if the notification is made in good faith.
(h) Nothing in the article may be construed to require a practitioner to access the West Virginia Controlled Substances Monitoring Program Database except as provided in §60A-9-5 of this code.
(i) The Board of Pharmacy shall provide an annual report on the West Virginia Controlled Substances Monitoring Program to the Legislative Oversight Commission on Health and Human Resources Accountability with recommendations for needed legislation no later than January 1 of each year.
§60A-9-5a. Practitioner requirements to access database and conduct annual search of the database; required rulemaking.
(a) All practitioners, as that term is defined in §60A-2-101 of this code who prescribe or dispense Schedule II, III, or IV controlled substances shall register with the West Virginia Controlled Substances Monitoring Program and obtain and maintain online or other electronic access to the program database: Provided, That compliance with the provisions of this subsection must be accomplished within 30 days of the practitioner obtaining a new license: Provided, however, That the Board of Pharmacy may renew a practitioner’s license without proof that the practitioner meets the requirements of this subsection.
(b) All persons with prescriptive or dispensing authority and in possession of a valid Drug Enforcement Administration registration identification number and who are licensed by the Board of Medicine as set forth in §30-3-1 et seq. of this code, the Board of Registered Professional Nurses as set forth in §30-7-1 et seq. of this code, the Board of Dental Examiners as set forth in §30-7-1 et seq. of this code, the Board of Osteopathic Medicine as set forth in §30-14-1 et seq. of this code, the West Virginia Board of Veterinary Medicine as set forth in §30-10-1 et seq. of this code, and the West Virginia Board of Optometrists as set forth in §30-8-1 et seq. of this code, upon initially prescribing or dispensing any Schedule II controlled substance, any opioid or any benzodiazepine to a patient who is not suffering from a terminal illness, and at least annually thereafter should the practitioner or dispenser continue to treat the patient with a controlled substance, shall access the West Virginia Controlled Substances Monitoring Program Database for information regarding specific patients. The information obtained from accessing the West Virginia Controlled Substances Monitoring Program Database for the patient shall be documented in the patient’s medical record maintained by a private prescriber or any inpatient facility licensed pursuant to the provisions of chapter 16 of this code. A pain-relieving controlled substance shall be defined as set forth in §30-3A-1 of this code.
(c) The various boards mentioned in §60A-9-5(b) of this code shall promulgate both emergency and legislative rules pursuant to the provisions of §29A-3-1 et seq. of this code to effectuate the provisions of this article.
§60A-9-6. Promulgation of rules.
The state Board of Pharmacy shall promulgate legislative rules to effectuate the purposes of this article in accordance with the provisions of chapter twenty-nine-a of this code.
§60A-9-7. Criminal penalties; and administrative violations.
(a) Any person who is required to submit information to the state Board of Pharmacy pursuant to the provisions of this article who fails to do so as directed by the board is guilty of a misdemeanor and, upon conviction thereof, shall be fined not less than $100 nor more than $500.
(b) Any person who is required to submit information to the state Board of Pharmacy pursuant to the provisions of this article who knowingly and willfully refuses to submit the information required by this article is guilty of a misdemeanor and, upon conviction thereof, shall be confined in a county or regional jail not more than six months or fined not more than $1,000, or both confined and fined.
(c) Any person who is required by the provisions of this article to submit information to the state Board of Pharmacy who knowingly submits thereto information known to that person to be false or fraudulent is guilty of a misdemeanor and, upon conviction thereof, shall be confined in a county or regional jail not more than one year or fined not more than $5,000, or both confined and fined.
(d) Any person granted access to the information required by the provisions of this article to be maintained by the state Board of Pharmacy, who shall willfully disclose the information required to be maintained by this article in a manner inconsistent with a legitimate law-enforcement purpose, a legitimate professional regulatory purpose, the terms of a court order or as otherwise expressly authorized by the provisions of this article is guilty of a misdemeanor and, upon conviction thereof, shall be confined in a county or regional jail for not more than six months or fined not more than $1,000, or both confined and fined.
(e) Unauthorized access or use or unauthorized disclosure for reasons unrelated to the purposes of this article of the information in the database is a felony punishable by imprisonment in a state correctional facility for not less than one year nor more than five years or fined not less than $3,000 nor more than $10,000, or both imprisoned or fined.
(f) Any practitioner who fails to register with the West Virginia Controlled Substances Monitoring Program and obtain and maintain online or other electronic access to the program database as required in subsection (a), section five-a, article nine of this chapter, shall be subject to an administrative penalty of $1,000 by the licensing board of his or her licensure. All such fines collected pursuant to this subsection shall be remitted by the applicable licensing board to the Fight Substance Abuse Fund created under section eight of this article. The provisions of this subsection shall become effective on July 1, 2016.
(g) Any practitioner or dispenser who is required to access the information contained in the West Virginia Controlled Substances Monitoring Program database as set forth in subsection (a), section five-a of this article and fails to do so as directed by the rules of his or her licensing board shall be subject to such discipline as the licensing board deems appropriate and on or after July 1, 2016, be subject to a $100 administrative penalty per violation by the applicable licensing board. All such fines collected pursuant to this subsection shall be transferred by the applicable licensing board to the Fight Substance Abuse Fund created under section eight of this article.
(h) Lack of available internet connectivity is a defense to any action brought pursuant to subsections (d) or (f) of this section.
§60A-9-8. Creation of Fight Substance Abuse Fund.
There is hereby created a special revenue account in the state treasury, designated the Fight Substance Abuse Fund, which shall be an interest-bearing account. The fund shall consist of all money received from whatever source to further the purpose of this article. The fund shall be administered by the West Virginia Bureau for Public Health to provide funding for substance abuse prevention, treatment, treatment coordination, recovery, and education. Any sums of money remaining in the fund at the close of a fiscal year shall be carried forward for use in the next fiscal year. Fund balances shall be invested with the state’s consolidated investment fund and any and all interest earnings on these investments shall be used solely for the purposes that money deposited in the fund may be used pursuant to this article. There is created within the Office of the Secretary of the Department of Health and Human Resources the Grant Writer Pilot Project. The Secretary shall hire a person as a grant writer, who shall be placed within the Office of the Secretary. This person shall identify, application, and monitoring policies and procedures to increase grant applications and improve management and oversight of grants. The grant writer shall focus his or her abilities on obtaining grants concerning the prevention and treatment of substance abuse. The grant writer is not eligible for civil service. The department shall report to the Legislative Oversight Commission on Health and Human Resources Accountability on the implementation of the new grant policy; the number of grants obtained; and analysis examining the costs associated with obtaining a grant versus the federal money received.
§60A-9-9. Drugs of concern designation.
(a) The Board of Pharmacy may designate certain drugs as drugs of concern which must be reported to the database established pursuant to this article. The designation of a drug of concern shall be reserved for drugs that have a high potential for abuse. Whenever a medical services provider dispenses a drug of concern or whenever a prescription for a drug of concern is filled by: (i) A pharmacist or pharmacy in this state; (ii) a hospital, or other health care facility, for outpatient use; or (iii) a pharmacy or pharmacist licensed by the Board of Pharmacy, but situated outside this state for delivery to a person residing in this state, the medical services provider, health care facility, pharmacist or pharmacy shall, in a manner prescribed by rules promulgated by the Board of Pharmacy under this article, report the following information, as applicable:
(1) The name, address, pharmacy prescription number, and Drug Enforcement Administration controlled substance registration number of the dispensing pharmacy or the dispensing physician or dentist;
(2) The full legal name, address, and birth date of the person for whom the prescription is written;
(3) The name, address, and Drug Enforcement Administration controlled substances registration number of the practitioner writing the prescription;
(4) The name and national drug number of the drug of concern dispensed;
(5) The quantity and dosage of the drug of concern dispensed;
(6) The date the prescription was written and the date filled;
(7) The number of refills, if any, authorized by the prescription;
(8) If the prescription being dispensed is being picked up by someone other than the patient on behalf of the patient, information about the person picking up the prescription as set forth on the person’s government-issued photo identification card shall be retained in either print or electronic form until such time as otherwise directed by rule promulgated by the Board of Pharmacy; and
(9) The source of payment for the drug of concern dispensed.
(b) The penalties set forth in section seven of this article shall not apply to drugs listed as drugs of concern. Failure to report may be considered a violation of the practice act of the prescriber and may result in discipline by the appropriate licensing board.
(c) The Board of Pharmacy may promulgate emergency rules pursuant to the provisions of section fifteen, article three, chapter twenty-nine-a of this code to effectuate the provisions of this section.
Enhancing Healthcare Team Outcomes
Chronic pain is a significant condition that affects many millions of people and is an important public health concern with considerable morbidity and mortality and associated opiate drug diversion and misuse. Thus chronic pain is best managed with an interprofessional approach. Managing chronic pain requires an interprofessional team of healthcare professionals that includes a primary care physician, nursing team, pharmacist, and pain medicine specialists. Without proper management, the patient's quality of life can have a deleterious impact. The evaluation and treatment of such patients are paramount but in order to avoid drug diversion and misuse, health professionals must work as a team.
Chronic pain correlates with several severe complications, including severe depression and suicide attempts and ideation. The lifetime prevalence for chronic pain patients attempting suicide attempt was shown to be between 5% and 14%; suicidal ideation was approximately 20%[11] These complications often require psychiatric intervention and advanced pharmacological or interventional therapies. Severe symptoms must receive treatment immediately, leading to an increase in healthcare costs. It is of the utmost importance to identify the risk factors and perform a thorough assessment of the patient with chronic pain as well as monitor for progression of symptoms. A team approach is an ideal way to limit the effects of chronic pain and its complications.
- Evaluation of a patient with acute pain by the primary care provider to prevent the progression of chronic pain is the recommended first step.
- Conservative management of chronic pain should commence when symptoms are mild or moderate, including physical therapy, cognitive-behavioral therapy, and pharmacological management.
- A pharmacist or other expert knowledgeable in the medications frequently utilized to treat chronic pain should evaluate the medication regimen to include medication reconciliation to preclude any drug-drug interactions, and alert the healthcare team regarding any concerns.
- The patient should follow up with a primary care provider as well as pain specialists as necessary regularly to assess and effectively treat the patient's pain.
- Clinicians must address comorbid psychiatric disorders. This action may require the involvement of a psychiatrist, depending on the severity of the patient's symptoms.
- If symptoms worsen on follow up or if there is a concerning escalation of pharmacological therapy such as with opioids, a referral to a pain medicine specialist merits consideration.
- If the patient has exhausted various pharmacological and nonpharmacological treatment options, interventional procedures can be a consideration.
- If at any time the patient expresses concern for suicidal ideation or plan, an emergent psychiatric team should evaluate the patient immediately.
- Patients who have developed opioid dependence secondary to pharmacological therapy should be offered treatment, possibly referral for addiction treatment or detoxification if indicated. The patient should be put on a medication weaning schedule or possibly medications for the treatment for opioid dependence.
- Based on CDC recommendations, patients on high-dose opioid medications or patients with risk factors for opioid overdose (e.g., obesity, sleep apnea, concurrent benzodiazepine use, etc.) should receive naloxone at home for the emergent treatment of an unintentional overdose.
The interprofessional team should openly discuss and communicate clearly about the management of each patient so that the patient receives optimal care delivery. This area is where nurses and pharmacists can play a crucial role by helping verify patient compliance with the treatment plan and monitor for progress (or lack of) with the present treatment plan. Nurses and pharmacists can help monitor for adverse medication side effects, concerns regarding diversion or misuse of opioids, and communicate any areas of concern to the treating clinicians. Effective, open interprofessional communication is crucial in the optimal management of chronic pain [Level 2] and in minimizing the negative effects of chronic pain in the patient.
(Click Image to Enlarge)