Active Tuberculosis
- Article Author:
- Talha Jilani
- Article Author:
- Akshay Avula
- Article Author:
- Anoosh Zafar Gondal
- Article Editor:
- Abdul Siddiqui
- Updated:
- 8/10/2020 4:44:02 PM
- For CME on this topic:
- Active Tuberculosis CME
- PubMed Link:
- Active Tuberculosis
Introduction
Active tuberculosis is a multiorgan disease caused by primary infection or as a reactivation of latent tuberculosis. Accordingly, active tuberculosis could be primary tuberculosis or reactivation tuberculosis. Primary tuberculosis occurs when the immune system is unable to defend against the Mycobacterium tuberculosis bacterium (MTB) infection. Reactivation tuberculosis, as the name suggests, is the reactivation of contained mycobacterial infection. Reactivation Tb is the most common form of active tuberculosis, representing 90% of the cases. The lung is the most commonly involved organ, other organ systems commonly affected include the gastrointestinal system, the musculoskeletal system, the lymphoreticular system, skin, liver, and the reproductive system.[1]
The World Health Organization (WHO) estimates that, annually, around 8 million people develop active tuberculosis globally, and nearly 2 million people die from the disease. Of every 10 people infected with M. tuberculosis, one may develop an active infection sometime in their lifetime. The WHO reported in 2017 that the estimated global incidence rate for tuberculosis has decreased by 1.5% each year since 2000. However, despite these substantial gains and drastic global efforts to eradicate tuberculosis, the disease still accounts for significant morbidity and mortality worldwide. Developing countries like India, Pakistan, the Philippines, China, South Africa, Indonesia, and Nigeria experience the highest morbidity and mortality rates. When combined, these countries accounted for 64% of all tuberculosis-related deaths in 2016, according to the WHO.[2][3][4]
Etiology
Infection with Mycobacterium tuberculosis (alcohol and acid-fast bacillus) causes active tuberculosis. It is classified under the M. tuberculosis complex group, which includes four other mycobacteria that can cause active tuberculosis: M. canettii, M. microti, M. bovis, and M. africanum.[5][6][7]
M. tuberculosis is an obligate-aerobic, nonmotile, non-spore-forming, catalase-negative, and facultative intracellular bacteria. The high lipid content of M. tuberculosis gives it many unique clinical characteristics. These include resistance to several antibiotics and the ability to survive in many extreme conditions. It also takes a long time to divide (around 16 to 20 hours), a significantly slower rate compared to other bacteria, which usually takes less than an hour.
The organism has a poor reaction to Gram stain and, thus, is not classified as gram-positive or gram-negative. However, sometimes weakly positive cells are observed on Gram stain, referred to as “ghost cells.” As M. tuberculosis retains some stains even after being treated with solutions containing acids, hence it is considered an acid-fast bacillus. The Ziehl-Neelsen stain and the Kinyoun stain are most commonly used to identify M. tuberculosis. The test dyes the acid-fast bacilli bright red, which makes it distinct against a blue background.
Humans are the only known host in which M. tuberculosis naturally lives and reproduces. The organism is spread primarily as an airborne aerosol from an individual in the infectious stage of the disease, although transdermal and gastrointestinal (GI) transmission is also possible.
Epidemiology
Tuberculosis has a global distribution, and over two billion people (around 30% of the world's population) are suspected to be infected with M. tuberculosis. In 2003, the global incidence of tuberculosis reached its peak but had been declining slowly ever since. Most new cases of the disease in 2016 were reported from Asia (around 45%), followed by Africa (around 25%). The WHO reported in 2016 that approximately 10.4 million people became infected with tuberculosis, of which approximately 1.7 million died.[8][9][10]
Although present globally, the epidemiology of tuberculosis varies significantly depending on the region.
- India, sub-Saharan Africa, Micronesia, and the islands of Southeast Asia had the highest rates (100 per 100,000 people or higher)
- China, Eastern Europe, Central, and South America, and northern Africa had intermediate rates (26 to 100 cases per 100,000 people)
- The United States, Canada, Japan, Western Europe, and Australia recorded the lowest rates (less than 25 cases per 100,000 people)[11]
Although about 90% to 95% of the people infected with M. tuberculosis do not develop the active disease and remain asymptomatic, around 5% to 10% of those infected develop the disease. In 2012, this amounted to around 8.6 million cases of active tuberculosis worldwide. In the United States, tuberculosis is commonly seen among the immigrant population. Whereas infections with nontuberculous mycobacteria are not uncommon in the US, especially among the susceptible and immunocompromised patients, many times the clinical differentiation among M. tuberculosis and nontuberculous mycobacterium (NTM) becomes challenging as they possess similar clinical and pathophysiologic mechanisms. Nevertheless, it is important to distinguish among tuberculosis, and nontuberculous mycobacteria as the management is drastically different for both[12].
Young adults have the highest rates of active tuberculosis globally, but in developed countries, older individuals experience the highest rates of disease. Adults of all age groups are at risk of progressing to active disease. Risk factors in contracting active disease include [13]:
- Coinfection with HIV is 20 to 30 times more likely to develop active tuberculosis.
- Presence of other immunocompromised states, including immunosuppressive agents such as long-term corticosteroids and anti-TNF medications.
- Chronic lung diseases.
- Use of tobacco products.
- Malnutrition indicates a higher risk of progressing to active disease, making tuberculosis one of the major illnesses of poverty.
- Diabetes also has an increased risk of progressing to active tuberculosis and experience worse treatment outcomes.
- The use of alcohol (greater than 40 g per day).
- Intravenous drug abuse.
- Indoor pollution.
- Silicosis
- End-stage renal disease.
- Intestinal bypass surgery or gastrectomy.
- Chronic malabsorption syndromes.[11]
Pathophysiology
Inhaling the aerosolized droplets from an infected person is the principal mechanism through which tuberculosis spreads. Although tuberculosis most commonly causes a lung infection, it is a multisystemic disease and can present as variable pathologic findings. Subsequent deposition of the organism in the lungs can lead to a few possible outcomes, including:
- The immediate clearance of the organism from the body
- Primary disease: The immediate onset of active disease in the individual
- Latent infection
- Reactivation disease: The onset of an active disease many years after a period of latent infection
The body's ability to effectively restrict or eliminate the infective inoculum is dependent on the immune status of the individual, genetics, and whether exposure to the organism is primary or secondary. M. tuberculosis also possesses several virulence factors that make its elimination difficult for alveolar macrophages. These factors include the high mycolic acid content found in the bacterium's outer capsule, which makes phagocytosis difficult for alveolar macrophages. Other constituents of the bacterium's cell wall, such as the cord factor, could directly damage the alveolar macrophages. Several studies have demonstrated that M. tuberculosis also inhibits the formation of an effective phagolysosome, which limits and sometimes even prevents the elimination of the organisms. Other virulence factors include catalase-peroxidase, which helps resist the oxidative response of the host cell, and lipoarabinomannan, which helps induce cytokines and resist host oxidative stress.[14][15]
Most exposed individuals clear the infection with the aid of innate immunity. About 30-40% of exposed individuals become infected. Adaptive (T-Cell) immunity contains the infection in 95% of infected individuals as latent tuberculosis. About 5 % of patients develop primary active tuberculosis secondary to inadequate T-cell immunity. About 5-10% of patients with latent infections progress to active disease in their lifetime, most commonly in the initial two years. If untreated, half of those with the active disease die.[16]
The tubercle bacilli induce infection of the lungs after they are transported in droplets small enough to reach the alveolar space (around 5 to 10 microns). If the infection is not eliminated by the innate defense system of the host, the bacilli could proliferate inside alveolar macrophages, which could migrate away from the lungs and enter other tissues.[17]
Macrophages in the lungs produce chemokines and cytokines that attract other phagocytic cells, including neutrophils, monocytes, and other alveolar macrophages, which produce a nodular granulomatous structure known as a tubercle. If the continuous bacterial replication is not inhibited, the enlarging tubercle and bacilli could enter local draining lymph nodes. This causes lymphadenopathy, which is a characteristic manifestation of primary tuberculosis. A Ghon complex can develop if the lesion produced by the extension of the tubercle spreads into the lung parenchyma and lymph node. Bacteremia could also be seen in the initial infection.[15]
The tubercle bacilli will proliferate until an effective cell-mediated immune response develops. This usually takes about 2 to 10 weeks following initial infection in more than 90% of infected individuals. In the lungs, failure to mount an effective cell-mediated immune response and tissue repair could lead to extensive damage. Tumor necrosis factor-alpha, nitrogen intermediates, reactive oxygen, and the constituents of cytotoxic cells (perforin, granzymes) whose function it is to eliminate M. tuberculosis could also contribute to the collateral damage of the host and the development of caseating necrosis. Therefore, much of the tuberculosis pathology results from the infected host's immune response to the tubercle bacilli.[18]
Unchecked bacterial growth could lead to the hematogenous spread of bacilli and eventually disseminated tuberculosis. "Military tuberculosis" is the term for disseminated disease with lesions resembling millet seeds. The bacilli can also spread mechanically via erosion of the caseating lesions into the airways, at which time the host becomes infectious to other individuals. In the absence of treatment, the mortality rate is around 80%. The remaining patients could develop a chronic disease or recover. Chronic tuberculosis is characterized by recurrent episodes of healing by fibrotic changes surrounding the lesions and tissue breakdown. The complete spontaneous eradication of the tubercle bacilli is rare.[18]
The primary tuberculosis infection is usually located in the middle portion of the lungs and is referred to as the Ghon focus. The Ghon focus usually enters a state of latency in most individuals as latent tuberculosis. In the event of immunosuppression in the host, latent tuberculosis reactivates to active tuberculosis.[1]
Most individuals who develop tuberculosis do so after an extended latency period, often many years after the initial primary infection. This is known as reactivation disease or secondary tuberculosis. Individuals with latent infection but no underlying medical problems have a 5% to 10% risk of developing secondary tuberculosis in their lifetime. The location of the lesions of secondary tuberculosis is usually at the lung apices, and unlike primary disease, it tends to be localized. A small number of people could also develop secondary tuberculosis after getting reinfected with M. tuberculosis. Secondary tuberculosis can be differentiated from primary progressive tuberculosis by the presence of cavitation and the location of the lesion.[18]
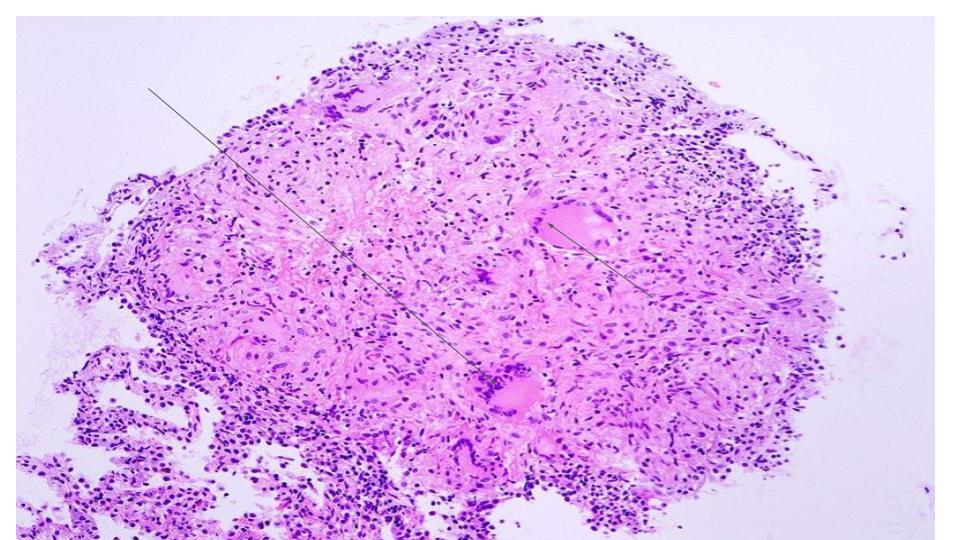
Histopathology
The diagnostic histopathological hallmark of tuberculosis is an epithelioid granuloma with central caseation necrosis. Tubercles are spherical nodules and have 3-4 cellular zones showing following features from the center to outwards:
- A central caseation necrosis
- An inner cellular zone containing lymphocytes, macrophages, epithelioid and giant cells
- An outer cellular zone of lymphocytes, immature macrophages, and plasma cells
- In healing lesions a rim of fibrosis
In caseation necrosis, there is low pH, low oxygen tension, and high fatty acid levels, all of which inhibit the growth of the tubercle bacillus and hence contain the infection.[19]
History and Physical
Pulmonary tuberculosis is the most common involvement in active tuberculosis. Extrapulmonary tuberculosis is seen in 10-42% of patients, depending on various host and bacterial factors. [20]
In pulmonary tuberculosis, the most commonly reported symptom is a chronic cough. Cough most of the time is productive, sometimes mixed with blood. Constitutional symptoms like fever, weight loss, lymphadenopathy, and night sweats are commonly reported. Extrapulmonary tuberculosis can affect any organ and can have a varied presentation.
When the clinical suspicion is high, further history should be obtained evaluating for risk factors of active tuberculosis:
- History of HIV infection - Degree of immunosuppression influence the clinical manifestations. [21]
- History of prior tuberculosis treatment
- History of a positive purified protein derivative of the tuberculin test result
- Emigration from or travel to an area where tuberculosis is endemic
- Contact with a person who has active TB
The presentation of secondary tuberculosis is different from that of primary progressive disease as the hypersensitivity, and tissue reaction is more severe in secondary tuberculosis. Primary tuberculosis often causes middle and lower lung field opacities associated with mediastinal adenopathy. Whereas secondary tuberculosis commonly involves upper lobes, causing opacities, cavities, or fibrotic scar tissue.
The active disease could also result in systemic dissemination of tubercles and manifest as miliary tuberculosis, which resembles millet-shaped lesions on radiographic images of the chest. Tuberculosis could also spread into the bowel, spine (Pott disease), or central nervous system (tubercular meningitis). Pleural tuberculosis is considered extrapulmonary tuberculosis. HIV co-infected patients with immunosuppression are at increased risk for extrapulmonary and disseminated tuberculosis.[22]
Physical examination depends on the organs involved. In the case of pulmonary TB, a patient can have crepitations, and bronchial breath sounds, especially over the upper lobes or affected area indicating cavity or consolidation. Signs of extrapulmonary TB are varied and can include:
- Lymphadenopathy
- Cutaneous lesions
- Pleural effusion
- Neurological deficit
- Confusion, coma
- Chorioretinitis
- Vertebral collapse[22]
Evaluation
Active tuberculosis is diagnosed by isolating Mycobacterium tuberculosis complex bacilli from bodily secretions. Any patient suspected of active tuberculosis is a public health risk for transmission and should be isolated with airborne precautions. In pulmonary tuberculosis, initial testing includes a chest X-Ray, sputum evaluation. Sputum evaluation includes Acid-Fast Bacilli smear (AFB smear), mycobacterial culture, and nucleic acid amplification testing (NAAT). The inability of sputum production can be an issue at times; in this instance, nebulized hypertonic saline can be used to induce sputum.
AFB Smear
CDC recommends the historical practice of obtaining three sputum samples with at least one early morning sample. Each specimen increases the sensitivity of testing.[23] The first-morning specimen increases sensitivity by 12%.[24] Sputum smear sensitivity could be increased by centrifugation or sedimentation.[25] Samples should be collected at least eight hours apart. ATS guidelines recommend at least 3ml of the sample, but the optimal volume of sputum is 5-10ml. [26] AFB smear is technically simple and can be performed in hours but cannot differentiate Mycobacterium tuberculosis from nontuberculous mycobacteria.
Genetic testing and NAAT
Nuclear amplification and gene-based tests represent a new generation of tools used for the diagnosis of tuberculosis. These tests enable the identification of the bacteria or bacteria particles by making use of DNA-based molecular techniques. These techniques are faster and allow accelerated diagnosis with high precision. Confirmation of the tuberculosis infection could be made in a few hours compared to the days or weeks it usually takes to wait for a standard culture. These tests are especially important among immunocompromised patients for whom there is a high rate of false-negative results. A few of the molecular-based tests such as DR-MTB and GeneXpert also allow for the identification of multiple-drug-resistant tuberculosis infections. A positive NAAT testing on a single sputum sample is considered sufficient for the diagnosis of Active Tuberculosis irrespective of AFB smear results. On occasions of negative AFB smear with intermediate to high suspicion of active tuberculosis, NAAT positivity can be used as presumptive TB. That being said, NAAT cannot be used to rule out pulmonary tuberculosis.
Sputum Mycobacterial Culture
Mycobacterial culture is the gold standard for diagnosis. Mycobacterial culture should be performed on both the solid and liquid medium. Liquid media culture can detect very low bacterial load and is considered a gold standard. Culture essential for drug susceptibility testing.[26] Solid media is less expensive but takes a longer time to grow the organism. Liquid media is expensive but more sensitive and grows organisms as early as 10-14 days.[27] Culture can differentiate MTB from NTM.
Drug susceptibility testing
Two methods of determining drug susceptibility are phenotypic and genotypic testing. [28] Genotypic testing is faster than the phenotypic methods. Microscopic observation of drug susceptibility (MODS) assay is a culture bases method to differentiate MTB from NTM and drug susceptibility to rifampin and isoniazid. Polymerase chain reaction (PCR) is another method to determine drug susceptibility. Numerous commercial test kits are available to determine drug susceptibility and are crucial in determining treatment course and duration.
Bronchoscopy
If all measures fail to obtain a sputum sample, a fibreoptic bronchoscopy with bronchioalveolar lavage can be performed with or without a transbronchial biopsy. Bronchoscopy can also be performed in high clinical suspicion with negative sputum studies and to rule out an alternative diagnosis.
Tissue biopsy
In the incidence of extrapulmonary tuberculosis, tissue biopsy of the affected organ is diagnostic.
Other
Apart from these, Tuberculin skin testing and/or interferon-gamma release assay should be performed depending on the situation. These tests should not be wholly relied upon in the diagnosis of active tuberculosis, but assist in diagnosis.
The Mantoux Test/ Tuberculin skin testing
The Mantoux test is a two-part test consisting of an intradermal injection of .1ml purified protein derivative and observing for induration 48-72 hours. The patient’s risk of exposure is taken into consideration when interpreting the result. Patients are then classified into three groups based on the size of the induration and the risk of exposure. These three groups include:
- Low risk: These patients have a minimal probability of exposure to tuberculosis. The Mantoux test is only considered positive if there is a significant induration of 15 mm or higher following the intradermal injection of purified protein derivative. People in this group include those with no history of travel to a tuberculosis-endemic region, no history of military service, no contact with a patient with a chronic cough, no history of steroid use, no known occupational exposure, are negative for HIV, and are not a resident of a tuberculosis-endemic country.
- Intermediate risk: These patients have an intermediate probability of exposure to tuberculosis. Their test results are considered positive if the measured induration is greater than 10 mm. People in this group include those who are residents of tuberculosis-endemic countries such as Asia, Latin America, and Africa, residents or workers of shelters, those who reside in overcrowded vicinities, and medical personnel.
- High risk: These patients have a significant probability of exposure to tuberculosis. Their test results are considered positive if the measured induration is greater than 5 mm. People in this group include those who are immunocompromised and cannot mount a sufficient immune response to the purified protein derivative test (HIV-positive patients, those on chronic steroids), patients with frequent exposure to those with a persistent cough, and patients with evidence of prior tuberculosis infection, such as a healed scar seen on X-ray.
A positive Mantoux test indicates exposure to tuberculosis or latent tuberculosis. However, this test lacks specificity, and patients require subsequent visits to interpret the result and a chest x-ray for confirmation of the disease. Although the test is considered relatively sensitive, false-positive results are seen with BCG vaccination. The Mantoux test should never be regarded as a confirmatory test.[29][30][31]
Interferon Gamma Release Assays
This is a far more specific screening test that is just as sensitive as the Mantoux test. It qualitatively assesses the level of inflammatory cytokines such as interferon-gamma. The advantage of this test, especially in those inoculated with the BCG vaccine, is that the test requires only a single blood draw, which negates the need for repeat visits by the patient to interpret results. Some disadvantages of this method include its high cost, the need for technical expertise to perform the test, high rates of false-positive, and false negatives.
Extrapulmonary tuberculosis
Diagnosing active extrapulmonary tuberculosis is similar to pulmonary tuberculosis. The definitive diagnosis is made by isolating M.Tuberculosis organisms from involved organ systems (e.g., pleural effusion, pericardial, peritoneal fluid, lymph node, bone marrow, blood, etc.). Additionally, biopsies of an involved organ system can also be used to isolate the organism. In the case of pleural involvement, the pleural biopsy is the gold standard. Tuberculosis Pleural effusion is exudative and rarely is positive for AFB. ATS/IDSA guidelines recommend checking for adenosine deaminase and interferon-gamma levels on any fluid collected on a patient suspected from extrapulmonary tuberculosis. [32]
Treatment / Management
Patients with suspected active tuberculosis should be isolated with airborne precautions, requiring negative pressure room either through a high-efficiency particulate air filter or by exhausting the air outside. Any person entering the room should be wearing high-efficiency airborne masks (Eg N-95) to filter the tubercle bacillus. Isolation should continue until collected sputum smears are negative for three consecutive determinations, usually after approximately 2 to 4 weeks of treatment. Unfortunately, these measures are neither practical nor possible in developing countries where tuberculosis is a public health problem.[33][34][35]
The treatment of an active tuberculosis infection requires a combination of drugs and includes intensive phase and the continuation phase. Monotherapy should never be used for the active disease to reduce the risk of the mycobacterium developing antibiotic resistance. First-line medications are the most commonly used regimens for active tuberculosis, including:
- Isoniazid: Used in combination with vitamin B6 to prevent neuropathies.
- Rifamycin: Patients should have baseline and follow-up liver function tests done as rifamycin is hepatotoxic.
- Ethambutol: Avoided in children whose visual acuity cannot be determined and monitored, as ethambutol can cause optic neuritis.
- Pyrazinamide: Patients should have periodic liver function tests, chest X-rays, serum uric acids, and sputum cultures done at 2 to 3 months and the completion of treatment.
The intensive phase includes a four-medication combination (isoniazid, rifampin, ethambutol, and pyrazinamide) is administered for two months, followed by a continuation phase consisting of a combination of isoniazid and rifampin for four months.
Directly observed therapy is recommended for patients receiving treatment. With this type of therapy, patients on the above regimens could be switched to 2 to 3 times per week dosing after completing an initial two weeks of daily dosing. Those taking medication two times per week must not miss any doses. Daily therapy should be prescribed for patients who are on self-administered medication. [36][37]
Patients diagnosed with active tuberculosis should have sputum analysis done for M. tuberculosis every week until sputum conversion is documented.
Second-line medications include:
- Injectable Aminoglycoside: Streptomycin, amikacin, and kanamycin.
- Injectable polypeptides: Viomycin and capreomycin
- Fluoroquinolones: Levofloxacin, gatifloxacin, ofloxacin, and moxifloxacin
- Others: Para-aminosalicylic acid, ethionamide, cycloserine, prothionamide, terizidone, linezolid, and thioacetazone.
Third-line anti-tuberculosis medications are drugs with variable but unproven efficacy against the disease. They are the last resort for total drug-resistant tuberculosis infections and include:
- Amoxicillin/clavulanic acid
- Clarithromycin
- Clofazimine
- Linezolid
- Imipenem/cilastatin
Drug resistance
- Monoresistant - Resistance to anyone TB treatment
- Poly-resistant - Resistance to two TB drugs (but not both isoniazid and rifampin)
- Multidrug resistance - Resistant to isoniazid and rifampin
- Extensively drug-resistant - Resistant to isoniazid and rifampin, plus resistance to any fluoroquinolone and 1 of the 3 injectable second-line drugs
Any patient that grows resistant MTB should be referred to an infectious disease expert. Multiple-drug-resistant tuberculosis infections are becoming increasingly common. A high-dose combination of the first-line and second-line drugs is being used to treat this condition.
Differential Diagnosis
The differential diagnosis consists of the following:
- Actinomycosis
- Histoplasmosis
- Blastomycosis
- Cat scratch disease
- Aspergillosis
- Lung abscess
- Nocardiosis
- Lung cancer
Toxicity and Side Effect Management
Side effects of anti-tuberculous drugs include:
Isoniazid
Liver injury (nausea, fatigue, vomiting, malaise, abdominal pain), nausea, rash, numbness, and tingling in extremities, headache
Rifamycin
Jaundice, red/orange discoloration of the urine and other secretions of the body, arthralgias, fever
Ethambutol
Visual dysfunction which includes blurring or decreased vision, and blindness, liver injury, headache, nausea
Pyrinzamide
nausea, painful or swollen joints, liver injury
Regular monitoring of liver function tests is essential, and in the case of elevated liver enzymes, depending on the severity, the medications should be discontinued. Second or third-line drugs can be used instead.[38]
Prognosis
The mortality rate for those with an active tuberculosis infection who do not receive adequate treatment is around 50%. Those at an increased risk for worse outcomes and possible death include:
- Young children (especially infants) and elderly patients
- Those who have a delay or do not receive proper treatment
- Patients who display an extensive spread of the disease on radiologic imaging
- Patients who have a severe respiratory compromise
- Those who are taking drugs or suffer from a disease that causes immunosuppression
Complications
Complications from active tuberculosis are frequently seen in patients with the risk factors mentioned above. Patients who do not receive proper or complete treatment for active disease are also at a higher risk of developing complications. Some complications caused by active disease include:
- Acute respiratory distress syndrome
- Extensive destruction of the lung
- Empyema
- Pneumothorax
- Disseminated tuberculosis infection (including tubercular meningitis)
- Bronchiectasis
- Fibrothorax
- Aspergilloma
- Hemoptysis[39]
Postoperative and Rehabilitation Care
All patients with TB need long-term follow up. This is to ensure that the disease is resolving and that the patient is compliant. As the treatment is long term, compliance with drug therapy can be low, and hence, the pharmacist may be involved in direct observation therapy.[40]
Consultations
- Infectious disease
- Thoracic surgeon if the patient develops complications
- Pulmonologist
Deterrence and Patient Education
The patient should be educated about compliance, as tuberculosis requires long term treatment. If non-compliance is a concern, "directly observed therapy" should be implemented as it is known to have better outcomes in patients, having difficulty adhering to the medication regimen.[41]
Educating the patient about potential side effects of treatment is of utmost importance. They should be counseled about monitoring, which should be done at least once per month to ensure there are no signs of toxicity, such as liver injury. Signs of liver injury must also be discussed, which include loss of appetite, vomiting, dark-colored urine, jaundice, abdominal pain, or fatigue. The patient needs to immediately stop taking the medication if any of these signs develop and should notify their healthcare provider.
Post-partum women are at an increased risk of side effects.[42]
Enhancing Healthcare Team Outcomes
Tuberculosis is a serious infection with the potential to spread. Whenever a case of tuberculosis is identified, the public health department must be notified. Untreated tuberculosis carries a mortality rate of more than 50%. More important, it can lead to devastating complications. Thus, the treatment of tuberculosis involves an interprofessional team approach that includes the pulmonologist, an infectious disease expert, a thoracic surgeon, and a public health nurse or clinician. Besides treatment, patient education is vital. The nurse and pharmacist should educate patients on the BCG vaccine, especially in children. The pharmacist is critical for direct observation therapy and ensuring compliance to treatment, and reporting back to the physicians, nurse practitioner, or physician assistant managing the patient if compliance is lacking. Further, since patients are treated as outpatients, the pharmacist is in a position to note the presence of any adverse effects of drug therapy, which also needs to be reported to the interprofessional team. The social worker should be involved to ensure that the patient has adequate financial resources for treatment. The patient needs close follow up with serial monitoring by the clinicians to ensure that the treatment is working. The patient should be urged not to get pregnant while on therapy, but if pregnancy does happen, the obstetrician and the infectious disease expert should be involved in the care. The infection control nurse should assist in educating the patient and family and assure that the medications are taken for their full course, providing followup and reporting to the team.
The radiologist should be involved as regular imaging studies are required to determine the response to treatment. Only through open communication between the team members can the morbidity and mortality rates of tuberculosis be reduced. [43][44][45](Level V)
Outcomes
The treatment of tuberculosis is slow, and full resolution can take months. Despite adequate therapy, recurrence rates vary from 2-12%. The recurrences usually occur with the first 12 months of therapy and may be due to reinfection or low compliance with drug therapy. Poor prognostic markers for the infection include immunocompromised state, extrapulmonary involvement, advanced age, and a history of prior infection. More important, complications of tuberculosis are also common and may include fibrothorax, collapsed lung, empyema, and massive hemoptysis. [3][46](Level V)
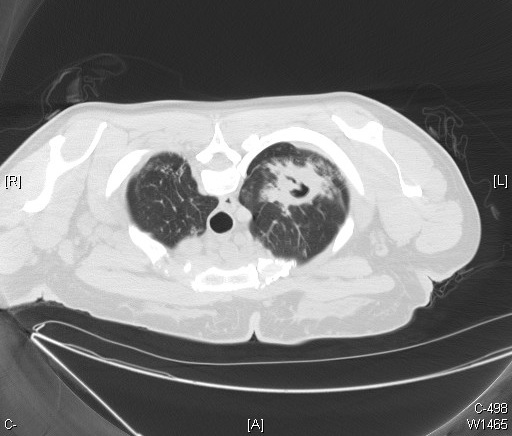
(Click Image to Enlarge)

An anteroposterior X-ray of a patient diagnosed with advanced bilateral pulmonary tuberculosis. This AP X-ray of the chest reveals the presence of bilateral pulmonary infiltrate (white triangles), and „caving formation“ (black arrows) present in the right apical region. The diagnosis is far-advanced tuberculosis.
Contributed by The Centers for Disease Control and Prevention (PD-USGov-HHS-CDC)
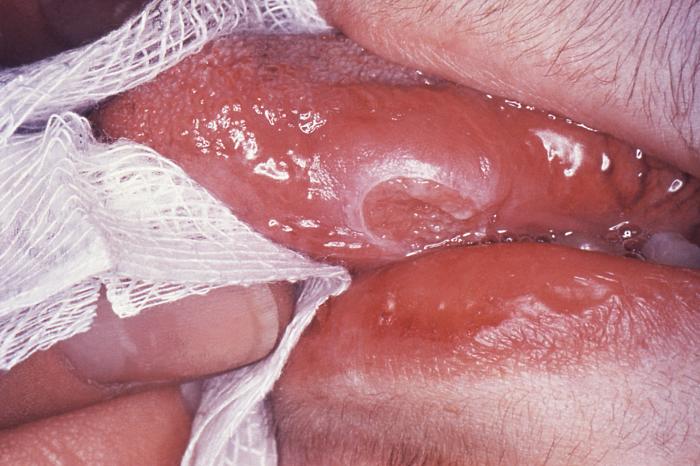
(Click Image to Enlarge)

This technician is in the process of correctly placing a Mantoux tuberculin skin test in this recipient’s forearm, which will cause a 6mm to10mm wheal, i.e., a raised area of skin surface, to form at the injection site. The Mantoux tuberculin skin test is used to evaluate people for latent tuberculosis (TB) infection. In the United States, this skin test consists of an intradermal injection of exactly one tenth of a milliliter (mL) of tuberculin, which contains five tuberculin units. Correct placement of this intradermal injection involves inserting the needle bevel slowly at a 5° to 15° angle. The needle bevel is advanced through the epidermis, the superficial layer of skin, approximately 3mm so that the entire bevel is covered and lies just under the skin surface. A tense, pale wheal that is 6mm to 10mm in diameter appears over the needle bevel.
Contributed by Wikimedia Commons, CDC, Greg Knobloch (PD-USGov-HHS)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)