Physiology, Afterload Reduction
- Article Author:
- Philip LaCombe
- Article Author:
- Muhammad Ali Tariq
- Article Editor:
- Sarah Lappin
- Updated:
- 5/4/2020 10:06:01 PM
- For CME on this topic:
- Physiology, Afterload Reduction CME
- PubMed Link:
- Physiology, Afterload Reduction
Introduction
The systolic performance of the heart is determined by 3 factors: preload, afterload, and contractility. The direct relationship between preload and cardiac output was formulated in the early 1900s based on the work of Otto Frank and Ernest Starling. It led to the well-known Frank-Starling curves. Gordon et al. helped to elucidate the underlying mechanism for this phenomenon in their 1966 experiments involving sarcomere length-tension relationships. During this same period, extensive research demonstrated an inverse relationship between afterload and systolic performance, which is accepted today. This means that cardiac output decreases as the afterload on the heart increases and vice versa. Despite this simple concept, there has been substantial controversy over the best way to represent cardiac afterload.
The afterload of any contracting muscle is defined as the total force that opposes sarcomere shortening minus the stretching force that existed before contraction. Applying this definition to the heart, afterload can be most easily described as the "load" against which the heart ejects blood. The load on individual fibers can be expressed as left ventricular wall stress, which is proportional to [(LV Pressure x LV Radius)/ LV wall thickness], or [(P x r)/h]. However, the true equation is complex because it depends on the shape of the cardiac chamber, which is affected by several factors that are changing over time. Therefore, afterload cannot be represented by a single numerical value or described only regarding pressure. Arterial pressure (diastolic, mean, or systolic) is frequently used as a surrogate measure, but perhaps the best available techniques involve measuring systemic arterial resistance by various invasive and noninvasive methods. Several mathematical models have been developed using arterial impedance and pressure-flow relationships to characterize afterload better, but these are complex and less often utilized in practice. The inverse relationship between afterload and cardiac output is important in understanding the pathophysiology and treatment of several diseases, including aortic stenosis, systemic hypertension, and congestive heart failure.[1][2][3]
Function
The afterload is the amount of pressure that the heart needs to exert to eject the blood out if it during the contraction. This is recorded as the systolic pressure of the heart. The changes in the afterload affect the stroke volume, end-systolic volume, end-diastolic volume, and left ventricular end-diastolic pressure. Afterload is increased due to an increase in systemic vascular resistance and aortic pressure increase. An increase in the afterload leads to a decrease in the stroke volume of the heart and an increase in the end-systolic volume. This also affects the cardiac output of the heart indirectly due to a reduction in the stroke volume of the heart. A Frank-Starling curve gives a relationship between stroke volume and left ventricular end-diastolic pressure. The pressure-volume loops explain the effects of afterload on the end-systolic volume and the end-diastolic volume.[4][5]
Mechanism
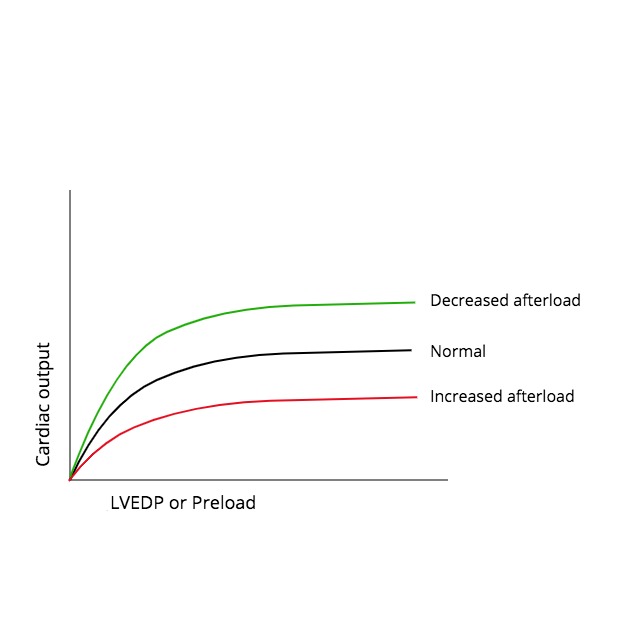
The relationship between afterload and cardiac output is somewhat intuitive as one would expect the flow to increase as the load against which the heart contracts decreases. Several researchers during the 1960s and 1970s sought to develop this understanding at the cellular level. Experiments by Sonnenblick on isolated cat papillary muscle strips demonstrated that the extent and velocity of muscle shortening decrease as the load on the muscle is increased. A major limitation of this study was its basic design employing the use of isolated muscle strips. Monroe and French overcame this by using isolated whole-preparation dog hearts to show an inverse relationship between peak aortic flow and arterial impedance. Ross et al. took this one step further and examined the effects of changing LV afterload in anesthetized dogs by injecting or withdrawing blood from the aorta in between systolic contractions. They reported similar findings to the previous studies giving further support for an inverse relationship between afterload and cardiac output due to alterations in sarcomere shortening. Figure 1 is a graphic representation of the effect of increases or decreases in afterload on the cardiac output, which is illustrated by shifting the baseline Frank-Starling curve downward or upward, respectively.[6][7][8]
Clinical Significance
Conditions in which there are chronic elevations in afterload, such as aortic stenosis and systemic hypertension, generate a cascade of adaptive responses, which can be both beneficial and ultimately detrimental. Initially, cardiac output is maintained through various regulatory mediators that increase inotropy. However, the ventricle responds to chronic elevations in afterload by concentric hypertrophy, causing increased wall thickness and decreased chamber diameter. This reduces internal wall stress at the expense of ventricular compliance leading to diastolic dysfunction (heart failure with preserved ejection fraction), which can further deteriorate into systolic dysfunction (heart failure with reduced ejection fraction).[9][10]
Afterload reduction agents are an essential component in treating congestive heart failure with reduced ejection fraction as these patients have elevated systemic resistance due to the neurohormonal response to the decreased cardiac output. They are also frequently used in the management of systemic hypertension. These drugs typically act by dilating the arterial system, which reduces the total load on the contracting heart and increases systolic performance. The arterial dilators fall under the broader category of vasodilators, which consists of arterial, venous, and mixed acting drugs. Venous dilators reduce preload by pooling blood in the highly compliant venous system and are an important part of treating angina. The preload reducing properties of venodilators lead to a reduction in cardiac output and arterial pressure. Most drugs have mixed arterial and venous action, and the relative balance between these determines the effect on cardiac output.[11]
Several classes of vasodilators are frequently used in practice and deserve a brief mention of their mechanism:
- Ace Inhibitors are one of the first-line medications for the management of hypertension and improve mortality in congestive heart failure with reduced ejection fraction. Their exact mechanism in CHF is considered to be complex, but it is accepted that they inhibit the formation of angiotensin II, which decreases vascular tone and improves systolic function. Ace inhibitors also prevent the formation of aldosterone-mediated by angiotensin II, which decreases preload by reducing sodium and water reabsorption.
- Nitroglycerin, isosorbide dinitrate/ mononitrate, and sodium nitroprusside belong to a subclass of vasodilators known as nitrodilators that work by increasing nitric oxide (NO) within the vascular smooth muscle and play a pivotal role in the management of angina and myocardial infarction. NO activates guanylyl cyclase leading to the formation of cGMP, which induces vascular relaxation through several mechanisms: decreasing intracellular calcium, activation of potassium channels inducing hyperpolarization, activation of cGMP dependent protein kinase that activates myosin light chain phosphatase. Nitroglycerin and isosorbide dinitrate/ mononitrate are considered organic nitrates because enzymatic conversion is required to generate nitrogen oxide, and they act primarily on the venous system to reduce preload with limited arterial dilation. Sodium nitroprusside is slightly different in that it releases NO without undergoing enzymatic conversion. It acts primarily on the arterial resistance vessels, which provide useful afterload reducing properties in hypertensive emergency and severe heart failure.[12]
- Hydralazine provides powerful afterload reducing properties and is thought to act solely as an arterial dilator through direct action on the vascular endothelium leading to NO generation. The full mechanism of hydralazine is complex and not completely understood.
- Sympatholytics include alpha one selective (prazosin) and non-selective alpha-blockers (phenoxybenzamine), which are used in the management of primary hypertension or hypertensive emergency secondary to pheochromocytoma, respectively. Sympatholytics act to reduce afterload by inhibiting the binding of norepinephrine to post-junctional alpha receptors preventing them from causing smooth muscle contraction. The effect is more significant on the arterial system, but they do have some venodilating properties as well.
- Dihydropyridines calcium channel blockers also have an effect on the peripheral vessels. They act by inhibiting the movement of calcium ions into the vascular smooth muscle cells. This has a vasodilatory effect on the vessels leading to a decrease in the systemic vascular resistance of the heart.[13]