Amyloidosis
- Article Author:
- Jean Bustamante
- Article Editor:
- Syed Rafay Zaidi
- Updated:
- 8/8/2020 9:02:44 PM
- For CME on this topic:
- Amyloidosis CME
- PubMed Link:
- Amyloidosis
Introduction
Amyloidosis is a heterogeneous disease that results from the deposition of toxic insoluble beta-sheet fibrillar protein aggregates in different tissues. Amyloidosis can be acquired or hereditary. The disease can be localized or systemic. Amyloid can accumulate in the liver, spleen, kidney, heart, nerves, and blood vessels, causing different clinical syndromes, including cardiomyopathy, hepatomegaly, proteinuria, macroglossia, autonomic dysfunction, ecchymoses, neuropathy, renal failure, hypertension, and corneal and vitreous abnormalities.[1][2][3]
Previously multiple classification systems have been devised to classify different types of amyloidosis. In modern times it has been classified according to the chemical analysis of amyloid clinical entities. Amyloidosis can be classified according to systemic, hereditary, central nervous system, ocular, and localized etiology. However, the most common types are AL, AA, ATTR (amyloid transport protein transthyretin), and dialysis-related amyloidosis (beta2M type).
In AL amyloidosis, 'A' represents amyloid followed by the associated fibrillar protein, 'L' means light chain fragment or immunoglobulin light chain. In AA amyloidosis, the second A stands for the serum amyloid A protein.[4][5]
Etiology
The most common causes of amyloidosis are the immunoglobulin-light-chain relate amyloidosis (AL), ATTR amyloidosis, and reactive amyloidosis (AA) due to chronic inflammatory diseases like chronic infections and rheumatoid arthritis. AL amyloidosis is acquired and is caused by a small plasma cell clone that produces misfolded amyloidogenic light chains that deposit in different organs and tissues.[6][7]
AA amyloidosis is associated with various chronic inflammatory conditions, chronic or local microbial infections, and rarely with neoplasms. AA is the most common type of systemic amyloidosis. However, its incidence varies in different ethnic groups.[8]
Epidemiology
AL amyloidosis has an incidence of 1 case per 100,000 person-years in Western countries. In the United States, there are approximately 1275 to 3200 new cases per year. The annual proportion of new cases with AL is 78%.
Familial transthyretin-associated amyloidosis (ATTR) is a less common systemic type of amyloidosis with unknown incidence, but approximately 10% to 20% of diagnosed cases in tertiary centers are secondary to ATTR amyloidosis. Of these cases, seven percent are hereditary and result from mutated transthyretin (ATTRm) and approximately six percent represent the acquired age-related, wild-type ATTRwt amyloidosis. ATTRwt is seen more commonly in males and was formerly known as senile systemic amyloidosis. Secondary or AA amyloidosis represents 6% of all the amyloidosis cases diagnosed each year. AA amyloidosis is an acquired process and reactive due to chronic inflammation.[9][10]
Pathophysiology
Twenty-one different proteins have been identified as amyloidogenic agents. Polypeptides can adopt alternative misfolded states, making them prone to aggregation. There are multiple processes by which misfolding of protein precursors occur. The protein may be intrinsically likely to acquire a pathologic conformation with aging, as seen in patients with wild-type transthyretin senile systemic amyloidosis. This can also happen when there is a high serum concentration of protein precursors, as seen in long-term hemodialysis patients with increased levels of beta-2-microglobulin.
In hereditary amyloidosis, the replacement of single amino acids can lead to amyloidogenic misfolded proteins losing the biologic function of the native proteins and, in turn, aggregate. Proteolytic remodeling of beta-amyloid precursor proteins has been identified in Alzheimer's disease. In patients with AA amyloidosis, serum amyloid A, an acute-phase reactant protein deposits in different tissues.
The amyloidogenic variants of the various precursor proteins are thermodynamically less stable, resulting in tetramers dissociation into monomers in the case of transthyretin and destabilization of the tertiary structure leading to partially folded conformers in the case of lysozyme. Transthyretin monomers and lysozyme partially folded conformers have the propensity to aggregate and assemble into fibrils. Charged residues also play a role in modulating the aggregation using repulsive forces resulting in partial unfolding, rendering the protein susceptible to proteases attacks and the release of amyloidogenic polypeptides. This is the case of gelsolin, an amyloidogenic protein that causes systemic amyloidosis.
Low pH, increased temperatures, limited proteolysis, osmolytes, and metal ions can alter the tridimensional structure of proteins shifting the equilibrium towards the amyloidogenic state. The mechanism of tissue damage in amyloidosis involves alteration of tissue architecture, interaction with cell surface receptors, inflammation elicited by the amyloid protein deposition, oxidative stress, and apoptosis activation.[11][12][13]
Histopathology
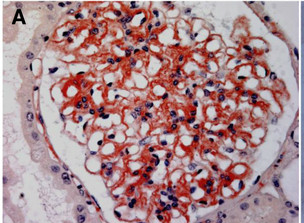
One diagnostic and differentiating feature of amyloidosis is the apple-green birefringence of amyloid on congo red staining. Apart from this, amyloid has an amorphous eosinophilic appearance, when viewed with hematoxylin and eosin staining. On x-ray diffraction analysis, it has a beta-pleated sheet structure.[14]
History and Physical
The clinical features of amyloidosis vary depending on which type of amyloid fibrils are responsible. Systemic amyloidosis (AA) can lead to heart failure with left ventricular hypertrophy on echocardiogram with standard or low voltage electrocardiogram. Hepatomegaly, nephrotic syndrome, macroglossia, orthostatic hypotension, ecchymosis, and autonomic and peripheral neuropathy can be present. Carpal tunnel syndrome, jaw claudication, and articular deposits of amyloid can also be a manifestation of systemic amyloidosis. In secondary amyloidosis (AA), hepatosplenomegaly, proteinuria, renal failure, and orthostasis can be seen. ATTR amyloidosis onset is during midlife and presents with peripheral and autonomic neuropathy, cardiomyopathy, and vitreous opacities. Amyloid beta-amyloidosis is localized to the central nervous system and presents as sporadic Alzheimer's disease and aging.[15][16]
Other physical exam findings that can lead to suspicion of amyloidosis are hypertrophied shoulder pads from amyloid deposition, amyloid purpura, and raccoon eyes secondary to factor-X deficiency in the case of AL amyloidosis and prolonged PT, PTT, that correct with mixing studies pointing towards the involvement of factor deficiencies in the common pathway of coagulation.[17]
Evaluation
Clinical suspicion, family history, and tissue biopsy establish the diagnosis.[18][19]
Tissue biopsy
- The subcutaneous abdominal fat aspirate is an easy and innocuous procedure that will stain Congo red positive with apple-green birefringence and has an 81% diagnostic sensitivity in AL amyloidosis.
- Biopsy of minor salivary glands can establish systemic amyloidosis diagnosis 60% of the times when fat aspirates are negative.
- Biopsy of the liver should be done by the transjugular route, since an amyloid-loaded liver may result in fatal bleeding.[20]
Plasma cell clone identification
Serum and urine electrophoresis with immunofixation and free light chains (FLC) should be requested to rule out plasma cell dyscrasia. When monoclonal light chains are not present, bone marrow biopsy can help establish the diagnosis by immunohistochemical staining. Immunofluorescence in situ hybridization (FISH) should be ordered along with a skeletal survey.
When plasma cell dyscrasia is ruled out, other types of amyloidosis should be considered. Tissue typing can be done by mass spectrometry, immune electron microscopy or immunohistochemistry. Transthyretin can be detected by isoelectric focusing on the serum which separates wild-type (or formerly known senile cardiac amyloidosis) and variant transthyretin.[21]
Gene sequencing
Should be done when hereditary amyloidosis has to be ruled out based on clinical grounds.
When transthyretin has not been identified, and patients have macroglossia and other typical organ involvement, AL should still be suspected despite plasma cell dyscrasia absence. ATTRwt is diagnosed by cardiac biopsy showing positivity to antibodies against normal transthyretin. Cardiac Scintigraphy with bone tracers can help differentiate AL amyloidosis which shows mild or no uptake from transthyretin amyloidosis which has strong uptake. This could potentially spare cardiac biopsy.
AA amyloidosis is considered when transthyretin and AL have been ruled out, and there are kidney involvement and neuropathy. Immunohistochemistry aids in the diagnosis of AA.
Organ involvement and staging of the disease should be established to design the treatment plan. For cardiac function, evaluation should include an echocardiogram with an assessment of strain, NT-proBNP, troponins, ECG, Holter ECG, and cardiac MRI should be solicited. For kidney function, evaluation 24-hour urinary protein and eGFR are needed. Liver function tests and imaging (ultrasound (US), MRI, or CT scan) can help with hepatic function assessment.
Treatment / Management
AL amyloidosis: This disease is treated most likely in the framework of clinical trials and depending on the risk stratification according to the Standard Mayo Clinic staging system.[22][23][24]
- Low-risk patients (NT-proBNP less than 5000 ng/L, troponins of less than 0.06 ng/ml, eGFR more than 50 ml/min per 1.73m, age less than 65 years, performance status of 0-2, NYHA class less than III, ejection fraction above 45%, systolic blood pressure above 90 mmHg standing and diffusion capacity of carbon monoxide (DLCO) above 90%). Low-risk patients receive autologous stem cell transplant (ASCT) with Melphalan 200mg/m2. Induction with cyclophosphamide, bortezomib, and dexamethasone should be considered if the bone marrow, plasma cell infiltration is more than 10% or if a patient refuses transplant. Post-transplant treatment with bortezomib increases the complete response (CR) rates, and if CR is not achieved, bortezomib and dexamethasone combination should be given.
- Intermediate risk patients (ineligible for ASCT and stage I-IIIa): Melphalan and Dexamethasone are the preferred regimens especially in the case of neuropathy or t(11;14) translocation. Cyclophosphamide with Bortezomib and dexamethasone combination is a stem cell sparing regimen preferred in patients with renal failure and with 1q21 gain. If noninvolved Free Light Chains (FLC) is above 180 mg/l, the preferred regimen is bortezomib, melphalan, and dexamethasone combination.
- High-risk patients (stage IIIb, NYHA class III or above): bortezomib can be preferred due to a rapid onset of action or low-dose combination regimens are preferred as well.
ATTR amyloidosis: Transthyretin is a protein predominantly synthesized in the liver. Upon liver transplant, mutant transthyretin disappeared from blood and neuropathy improvement was observed.
Supportive therapy: The treatment of systemic amyloidosis involves supportive therapy aiming to maintain the quality of life and prevent organ dysfunction. If patients are on a heart transplant waiting list, they should receive low-dose chemotherapy due to increased survival.
Splenectomy: An option with proper prior vaccination when there is severe factor-X deficiency leading to bleeding diathesis. Factor X deficiency is seen in 2.5% of patients with AL amyloidosis. Splenectomy is effective in patients who have splenomegaly, but not usually in patients with a normal size spleen.[25]
Differential Diagnosis
Chronic inflammatory conditions and systemic conditions are associated with amyloidosis. Systemic or secondary amyloidosis often coexists with immunoglobulin M associated disorders.[26]
The following is the differential diagnosis;
-
Familial Renal Amyloidosis
-
Immunoglobulin-Related Amyloidosis
-
Membranous Glomerulonephritis
-
Renal Vein Thrombosis due to amyloid
-
Cutis Verticis Gyrata
-
Mastocytosis
-
Nodular Localized Cutaneous Amyloidosis
-
Pseudoxanthoma Elasticum
Prognosis
The prognosis depends on the type of amyloidosis and the patient's response to treatment. Systemic amyloidosis if left untreated can be fatal. If the underlying chronic inflammatory condition is not picked up as a cause of amyloidosis or is misdiagnosed, amyloid aggregation usually accelerates. The survival rate depends on the organs affected. If the heart is involved, cardiovascular complications significantly increase morbidity and mortality.[27]
Complications
Amyloidosis affects multiple body organs and systems. Chronic deposition of amyloidosis leads to restrictive cardiomyopathy. Initially, cardiac involvement may cause angina, orthostatic hypotension or arrhythmias. Congestive heart failure accounts for the death of about 40% of patients that have primary systemic amyloidosis.[28]
Macroglossia can cause painful dysphagia. Infiltration of amyloid into blood vessels can cause leg or jaw claudication. Amyloid deposition in the skin can result in loss of scalp hair. Gastrointestinal tract infiltration with amyloid leads to hemorrhage that can cause malabsorption or can be fatal.
Consultations
Treatment of amyloidosis involves a multidisciplinary approach. Depending on the disease severity and the organs affected a clinical hematologist, cardiologist, nephrologist, pathologist, or other subspecialty specialists can be involved.
Enhancing Healthcare Team Outcomes
Amyloidosis is a heterogeneous disease that results from the deposition of toxic insoluble beta-sheet fibrillar protein aggregates in different tissues. Amyloidosis can be acquired or hereditary. The disease can be localized or systemic. Thus, it is best managed by an interprofessional team that includes an oncologist, hematologist, internist, nephrologist, and a neurologist. Amyloid can accumulate in the liver, spleen, kidney, heart, nerves, and blood vessels causing different clinical syndromes including cardiomyopathy, hepatomegaly, proteinuria, macroglossia, autonomic dysfunction, ecchymoses, neuropathy, renal failure, hypertension, and corneal and vitreous abnormalities. This disease is treated most likely in the framework of clinical trials and depending on the risk stratification according to the Standard Mayo Clinic staging system.[29]
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)