Anatomy, Anatomic Dead Space
- Article Author:
- Michael Quinn
- Article Author:
- Kayla St Lucia
- Article Editor:
- Alessandra Rizzo
- Updated:
- 10/30/2020 8:40:52 AM
- For CME on this topic:
- Anatomy, Anatomic Dead Space CME
- PubMed Link:
- Anatomy, Anatomic Dead Space
Introduction
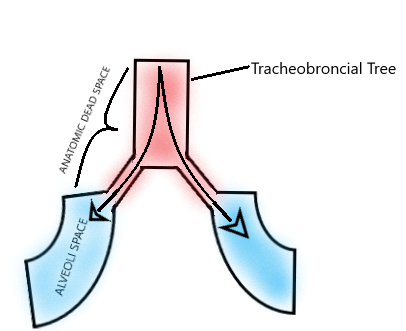
Dead space of the respiratory system refers to the space in which oxygen (O2) and carbon dioxide (CO2) gasses are not exchanged across the alveolar membrane in the respiratory tract. Anatomic dead space specifically refers to the volume of air located in the respiratory tract segments that are responsible for conducting air to the alveoli and respiratory bronchioles but do not take part in the process of gas exchange itself. These segments of the respiratory tract include the upper airways, trachea, bronchi, and terminal bronchioles. On the other hand, alveolar dead space refers to the volume of air in alveoli that are ventilated but not perfused, and thus gas exchange does not take place.[1][2][3]
Physiologic dead space (VDphys) is the sum of the anatomic (VDana) and alveolar (VDalv) dead space.
- VDphys = VDana + VDalv (L)
Dead space ventilation (VD) is then calculated by multiplying VDphys by the respiratory rate (RR).
- VD = VDphys x RR (L/min)
Total ventilation (VE) is, therefore, the sum of alveolar ventilation (Valv) and VD.
- VE = Valv + VD (L/min)
Enghoff's equation compiles these variables with PaCO2, tidal volume (TV), and expired CO2 (PECO2). It is then implied that VDphys/VT represents the portion of a tidal volume that does not participate in gas exchange.
- VDphys/VT = (PaCO2 - PECO2)/PaCO2
Dead space has particular significance in the concept of ventilation (V) and perfusion (Q) in the lung, represented by the V/Q ratio. Alveoli with no perfusion have a V/Q of infinity (Q=0), whereas alveoli with no ventilation have a V/Q of 0 (V=0). Therefore, in situations (i.e., V/Q =infinity) in which the alveoli are ventilated but not perfused, gas exchange cannot occur, such as when pulmonary embolism increases alveolar dead space.
Structure and Function
The function of a seemingly wasteful design for ventilation that includes dead space is as follows:
- Carbon dioxide is retained, resulting in bicarbonate-buffered blood and interstitium.
- Inspired air is raised or lowered to body temperature, increasing the affinity of hemoglobin for O2, and improving O2 uptake.
- Particulate matter is trapped in the mucus that lines the conducting airways, allowing it to be removed by mucociliary transport.
- Inspired air is humidified, thus improving the quality of airway mucus.
Physiologic Variants
Alveolar dead space typically is negligible in a healthy individual. Anatomic, and therefore physiological, dead space normally is estimated at 2mL/kg of body weight and comprises 1/3 of the TV in a healthy adult patient; it is even higher in pediatric patients. Effectively, 1/3 of a TV of inhaled air is rebreathed due to dead space. At the end of expiration, the dead volume consists of a gas mixture high in CO2 and low in O2 compared to ambient air.
End-expiratory dead volume: 5-6% CO2, 15-16% O2; Ambient air: 0.04% CO2, 21% O2
Numerous physiologic factors can influence dead space:
- Respiratory Cycle: Inhalation increases bronchial diameter and length, effectively increasing the anatomic dead space. Likewise, exhalation decreases the amount of anatomic dead space by "deflating" the bronchial tree.
- Positioning: Dead space decreases with the supine position and increases during a sitting position. The upright position allows a mismatched ratio of ventilation (V) and perfusion (Q) to occur, in which the apices of the lungs can not be as well perfused as ventilated (due to gravity's greater effect on blood than air), so wasted ventilation occurs and effectively increases dead space volume.
- Sleep: Anatomic dead space is believed to decrease during sleep and be the primary physiologic cause of observed decreases in tidal volume, minute ventilation, and respiratory rate during sleep.
- Maxilla: Variation also can occur in patients with maxillary defects or those who have undergone maxillectomy procedures. These patients have an increased anatomic dead space due to communication between the nasal and oral cavities, ultimately affecting respiratory function.
Surgical Considerations
In patients with disease-free lungs who are undergoing general anesthesia for procedures non-affective of the thoracic cavity or diaphragm, dead space and compliance of the lungs has enabled physicians to tailor patients' PEEP to optimal levels, with the reasoning that the point of minimum dead space with maximum compliance represents the point at which the maximum amount of alveoli are opened for ventilation. Increasing VD, however, can signify that alveoli may be over-distending from overly-aggressive ventilation parameters. Lung recruitment maneuvers in adjunct to PEEP in mechanical ventilation has been shown to significantly increase functional residual capacity, compliance, and PaO2 with decreases in dead space compared to PEEP alone.[4][5][6]
Clinical Significance
Dead space can be affected by various clinical scenarios:
- Lung Disease: Emphysema destroys alveolar tissue and leads to air trapping and decreased diffusion surface area, thereby increasing dead space volume. Acute Respiratory Distress Syndrome (ARDS) creates disturbances in the pulmonary microvasculature, theoretically increasing dead space. However, it is poorly understood if these portions of the lung are ventilated sufficiently to be considered dead space. VDphys/VT measured by Enghoff's equation increases in ARDS; however, due to the ratio being reflective of V/Q changes, which occur in pulmonary shunting mechanisms (perfusion without ventilation).
- V/Q Mismatch/Decreased Perfusion: Perfusion to the alveoli is decreased in clinical scenarios such as pulmonary embolism and hypotension, increasing the V/Q ratio and creating dead space ventilation.
- Mechanical Ventilation: Tubing from the ventilator increases dead space volume by adding volume to the effective space, not participating in gas exchange.
- PEEP: Excessive PEEP can over-distend alveoli and result in lung barotrauma, increasing the dead space volume.
- Hypoxia: Bronchoconstriction and vasoconstriction from hypoxia decrease dead space volume.
- Anesthesia: Bronchodilation from anesthetic gases increases dead space volume.
Estimating the dead space can be of significant value in clinical situations for diagnostic, prognostic, and therapeutic value. Dead space is an integral part of volume capnography, which measures expired CO2 and dead space (VDphys/VT) on a breath-by-breath basis for efficient monitoring of patient ventilation. Despite that the VDphys/VT ratio measured by Enghoff's equation is adversely affected by pulmonary shunting in ARDS, VDphys/VT has been shown to be a significant predictor of mortality during early-phase acute respiratory distress syndrome (ARDS), and increases in the VDphys/VT ratio correlated with poorer patient outcomes. Measurement of this dead space provides a quantifiable indicator of overall lung function for physicians to assess throughout the course of ARDS patients' hospital course. PEEP, an integral part of ARDS ventilation management, can be titrated to a patient's specific need based on capnography and dead space monitoring, but this finding has not been consistently shown in multiple studies.
Physicians with patients suspected of pulmonary embolism can use dead space and capnography findings to exclude the diagnosis with elevated D-dimer, a sensitive but not specific test for an embolism. Furthermore, capnography can be used for periodic monitoring of thrombolysis treatment in pulmonary embolism by trending changes in dead space measurements. Dead space and capnography can prove to be useful tools, minimizing unnecessary tests by ruling out pulmonary embolism with simple capnography measurements.
Clearance of the anatomic dead space is believed to play a significant role in using nasal high flow cannulas. It is believed that high nasal flow allows dead space to be cleared more rapidly and subsequently decreasing the portion of dead space that is rebreathed, increasing alveolar ventilation.