
Neuroanatomy, Auditory Pathway
- Article Author:
- Diana Peterson
- Article Author:
- Vamsi Reddy
- Article Editor:
- Renee Hamel
- Updated:
- 8/10/2020 5:47:37 PM
- For CME on this topic:
- Neuroanatomy, Auditory Pathway CME
- PubMed Link:
- Neuroanatomy, Auditory Pathway
Introduction
The auditory system processes how we hear and understand sounds within the environment. It is made up of both peripheral structures (e.g., outer, middle, and inner ear) and brain regions (cochlear nuclei, superior olivary nuclei, lateral lemniscus, inferior colliculus, medial geniculate nuclei, and auditory cortex). Auditory brain circuits encode frequency, attenuation, location in space. Some circuits also process combinations of these properties to help individuals understand and correctly interpret sounds. Processing of auditory information changes continuously by descending feedback circuits based on altered environmental, attentional, and perceived importance of environmental cues. The following chapter provides a basic description of audition and auditory processing.
Structure and Function
Peripheral Auditory System: How sound reaches the brain.
Sounds are produced by energy waves. Energy waves travel through a medium by moving molecules. This causes increases and decreases in pressure (i.e., alternating compression and rarefaction) of air within the environment. The number of periods of compression and rarefaction within a specified amount of time is the frequency of a specific sound. We measure frequency in Hertz (Hz; cycles of compression and rarefaction per second). Humans typically hear within a frequency range of 20-20,000 Hz.
Sound waves reach the outer ear and travel down the external acoustic meatus to reach the eardrum (tympanic membrane). Contact between the eardrum and environmental pressure waves causes movement of the membrane. Movement of the tympanic membrane initiates vibration of 3 small bones within the middle ear: the malleus, incus, and stapes which transfer the vibration to the inner ear at the oval (vestibular) window (Figure 1A).
The 3 middle ear bones amplify this energy and transfer it into the cochlea. Within the cochlea, mechanical energy converts to electrical energy by auditory receptor cells (hair cells). This conversion occurs within the cochlea of the inner ear. The cochlea is a fluid-filled (perilymph) structure that spirals 2 ½ turns around a central pillar (modiolus). In cross-section, each aspect of the cochlea has 3 sections: the scala tympani, scala vestibule, and scala media (Figure 2). The scala tympani lies within the outer portion of the cochlea. It is continuous with the scala vestibule (lining the inner portion of the cochlea) at the helicotrema. Between these fluid-filled areas is the scala media (Figure 1B). Oscillation of the oval window induce waves through the scala tympani and then scala vestibule of the cochlea. Waves from these regions press against and transmit wave energy to the scala media through the basilar membrane (within the floor of the scala media).
The Organ of Corti resides on the basilar membrane inside the scala media. It houses mechanical receptor cells: 3 rows of outer hair cells and one row of inner hair cells. The base of these cells is embedded within the basilar membrane. At the apex of each cell, stereocilia connect to a second membrane (tectorial membrane) within the scala media (Figure 1B).
As the scala vestibule and scala tympani oscillate, the basilar membrane shifts with the tectorial membrane. This shift bends the stereocilia with respect to the cell body of the hair cells. Depending on the direction of the shift, the movement will mechanically open or closes potassium channels to facilitate activation or deactivation of the cell.
How the tectorial and basilar membranes move changes depending on the location within the cochlea. The anatomy of the region close to the oval window is stiffer and hair cell stereocilia shorter. Therefore, cells near the oval window (base of the cochlea) respond to high frequencies. As you move toward the apex of the cochlea, there is more flexibility within the cochlea and the stereocilia length is more than twice as long as hair cells at the base. [1] This shift in flexibility and altered anatomy influences how the basilar and tectorial membranes move and cause the hair cells to respond to lower frequencies. [2] In this way graded flexibility allows hair cells within the cochlea to respond to a specific range of frequencies from high at the base to low at the apex of the cochlea. This arrangement of cells is called a tonotopic gradient.
Unlike other cells within the brain, hair cells within the Organ of Corti of the cochlea do not have axons. Neurons within the spinal ganglion have peripheral axons that synapse at the base of the hair cell soma. These axons make up the auditory nerve (Figure 1B). Most (90%) of auditory nerve fibers receive their input from the inner hair cells. [2] Thus, the inner hair cells facilitate a majority of auditory processing.
Outer hair cells synapse on only 10% of the spiral ganglion neurons. These neurons are special in that they can contract the length of their cell body which alters the stiffness of the basilar membrane. This form of stiffening can dampen the excitation of hair cells and thus alter what sound transmits through the auditory system. [3] Because the outer hair cells receive input from cortex, the cortex can start these changes to protect the health of hair cells in the presence of loud environments. [4][5] One example would be when an individual goes to a loud concert. Cortical feedback would initiate conformational changes to the outer hair cells to decrease movement within the cochlea (i.e., dampen the noise). When the individual leaves the concert, they may experience a loss of normal hearing for a few minutes and then resume normal hearing function. This delay is caused by the time needed for the descending circuits to reset anatomical morphology for the optimal audition in the new quieter environment.
Central Auditory System
Information from the peripheral auditory system reaches central auditory nuclei via the auditory nerve. The auditory nerve transmits auditory information up a series of nuclei to the cortex where perception occurs. These nuclei include 1) cochlear nucleus, 2) superior olivary nuclei, 3) lateral lemniscus, 4) inferior colliculus, and 5) medial geniculate nuclei. [6] Auditory information ascending through the auditory pathways start at the auditory nerve. These nerves synapse within the cochlear nucleus. A majority of auditory information is then transmitted through crossing fibers into the superior olivary complex. From there, the information ascends through the contralateral side of the brainstem and brain to the cortex (Figure 1C). It is of note that a significant number of neurons within the auditory system have crossing fibers at every level of the auditory system (Figure 1D). This is likely due to the need for both ipsilateral and contralateral information for many aspects of auditory processing. Therefore, all levels of the central auditory system receive and process information from both the ipsilateral and contralateral sides.
Types of Processing:
Different aspects of environmental sounds (e.g., attenuation: how loud the sound is; location in space; frequency, and combination sensitivity) are processed in each of the central auditory areas. Most of the auditory nuclei throughout the brain are tonotopically arranged. In this way, auditory signals ascending to the cortex can preserve the frequency information from the environment. [6]
Attenuation (the intensity of a sound), is processed within the auditory system by neurons that fire action potentials at different rates based on the sound intensity. Most neurons respond by increasing their firing rate in response to increased attenuation. More specialized neurons respond maximally to environmental sounds within specific intensity ranges. [6]
The brain processes the location of a sound in space by comparing differences in attenuation and timing of inputs from both ears within the superior olivary complex. If a sound is directly midline (i.e., front or back of the head), it would reach both ears at the same time. If it is to the right or left of midline, a temporal delay occurs between the inputs for the two ears. Within the superior olivary complex, specialized neurons receive input from both ears and can code for this temporal delay (i.e., binaural processing). [6]
Combination-sensitive neurons are another subset of neurons within the auditory system that have either enhanced or inhibited responses specifically to 2 or more sounds with a specific temporal delay. Combination-sensitive neurons are located within the inferior colliculus, lateral lemniscus, medial geniculate, and auditory cortex. [7][8][9][10][11] Because most sounds in the environment are not pure tones, these types of combination-sensitive neurons are thought to facilitate the enhancement of processing for combinations of sounds that may be important to the individual (e.g., speech, communication sounds). [12]
Descending Circuits
It was once thought that auditory processing was a simple relay from the environmental signals up to the cortex. We now know that there is a significant descending system of circuits within the auditory system that helps to modulate auditory processing at every level. The auditory cortex has bilateral direct projections back to the inferior colliculus, superior olivary complex, and cochlear nucleus. [13][14][15][16][17][18][19] These circuits contact neurons in these nuclei that project to every level of the central auditory system and to the cochlea (to modulate outer hair cells) within the peripheral auditory system. Connections between descending, ascending, and crossing fibers make the auditory system highly interconnected (Figure 1D). These descending circuits help to modulate auditory attention based on the relevance, attention, learned behaviors, and emotional state of an individual. Such higher-order functions originate from many regions of the brain (e.g., prefrontal cortex, hippocampus, nucleus basalis of Meynert, and limbic circuits) that have either direct and indirect connections with each other and auditory cortex. [20][21][22][23][24][25][26]
Embryology
The development of the cochlea originates at gestational day 4 from the surface ectoderm. It begins as an otic vesicle that develops into the membranous labyrinth of the inner ear. The dorsal aspect of the labyrinth develops into the utricle and semicircular ducts while the ventral aspect transforms into the cochlea and saccule.
The brain regions associated with central auditory processing develop with the various regions of the brain (auditory cortex: telencephalon; medial geniculate: diencephalon; inferior colliculus: mesencephalon; and the cochlear and superior olivary nuclei: rhombencephalon). These areas are fully functioning by birth. These regions are highly plastic. Therefore, auditory processing is in a constant form of change and development that lasts throughout life. [27]
Blood Supply and Lymphatics
Blood supply [28] (Standring, 2008):
External ear:
- Posterior auricular branch of the external carotid artery
Middle ear:
- Mastoid branches from the posterior auricular arteries
- Occipital arteries
- Deep auricular arteries
Inner ear:
- Anterior tympanic branch of the maxillary artery
- Stylomastoid branch of the posterior auricular artery
- Petrosal branch of the middle meningeal artery
- Labyrinthine artery (branch of the basilar or anterior inferior cerebellar artery)
Lymphatics of the ear:
External ear:
- Pre-auricular lymph nodes [29]
Middle ear:
- Retroauricular and junctional lymph nodes [30]
Inner ear:
- It is unclear whether the inner ear drains via a normal lymphatic system. Salt and Hirose [31] proposed that the inner ear drains diffusely through the perilymph and bone.
Muscles
There are no muscles within the auditory system. Two muscles (levator veli palatini and tensor veli palatini) assist with opening the auditory tube.
Physiologic Variants
Cauliflower ear: Repeated trauma can cause abnormalities of the outer ear. Trauma can induce a hematoma of the auricle in which blood accumulates between the perichondrium and auricular cartilage. This can distort the contours of the ear, impair blood flow to the cartilage, and lead to fibrosis and anatomical deformity (i.e., cauliflower ear). [32]
Otis media: Otitis media is an inflammation of the lining of the middle ear. These types of infections are common in children with more horizontally directed auditory tubes. As the skull develops, the tubes will slope in a lateral and caudal direction and allow better drainage. Therefore, otitis media is much more common in young children. The inflammation in these cases causes swelling and subsequent pressure on the tympanic membrane. In severe cases, the tympanic membrane can rupture leading to a decrease in the auditory acuity of affected individuals. [33]
Surgical Considerations
To facilitate recovery of recurrent otitis media and prevent scarring from a ruptured tympanic membrane, surgeons can insert drainage tubes into the tympanic membrane.
Clinical Significance
Acoustic Neuroma: The auditory nerve is located at the junction between the pons, medulla, and cerebellum (Figure 1D). This is the locus of a tumor called an acoustic neuroma. Acoustic neuromas grow slowly. [34] As it enlarges, it can put pressure on surrounding cranial nerves (e.g., Auditory VIII, Facial VII, and Glossopharyngeal IX), the cerebellum and brainstem. Initial symptoms include decreased hearing. As the tumor gets larger, it may include additional symptoms: (e.g., VIII: tinnitus, vertigo, nystagmus; VII: facial drooping, decreased corneal reflex; IX: hoarseness and dysphagia; Cerebellum: ataxia and dysarthria). The symptoms will present ipsilateral to the side of the tumor. [35]
Tinnitus: Tinnitus is the perception of a sound (typically a ringing or buzzing) that is not present in the environment. The perception is induced by a hyper-excitation within a specific frequency region of the auditory cortex. [36][37] Patients with tinnitus may complain of a lack of ability to differentiate sounds or conversations in noisy environments. Depending on the severity, it can also influence sleep, social, and emotional aspects of a patient’s life. [37][38]
Tinnitus typically presents after several years of repeated exposure to loud noises. It commonly has a gradual onset caused by repeated damage or stress to cochlear hair cells. However, a lack of input from the cochlear cells does not necessitate a lack of input to the cortex. Because neurons within the auditory system have multiple inputs, the central auditory regions receive input from other neurons about frequency, attenuation, and location in space of other sounds. They transmit these other signals to the auditory cortex within the affected denervation frequency range, thus producing a phantom perception.
There is no cure for tinnitus. Biofeedback therapies have helped in some cases, however, they do not eliminate the tinnitus perception. The biofeedback therapy uses one or more sounds played at or near the tinnitus perception of a patient. This form of feedback uses inhibitory limbic circuits to help depress the aberrant cortical excitation. [37] For individuals that have difficulty getting to sleep, playing white noise or other sounds immediately before sleep can also assist this population. [39]
Other Issues
While most of the population can respond to a typical hearing test in which they respond to sounds presented to different ears, a proportion of the population cannot respond due to lack of speech development (infants), disease, or trauma. A more universal test is the auditory brainstem response (ABR). This test does not require patient feedback. It records the summed changes in the electrical activity of auditory areas within the brainstem in response to auditory cues. Variations in activity from normal values show evidence for auditory dysfunction. Because the test shows values for multiple auditory nuclei, it also provides data for physicians to isolate regions of trauma or disease. [40]
(Click Image to Enlarge)
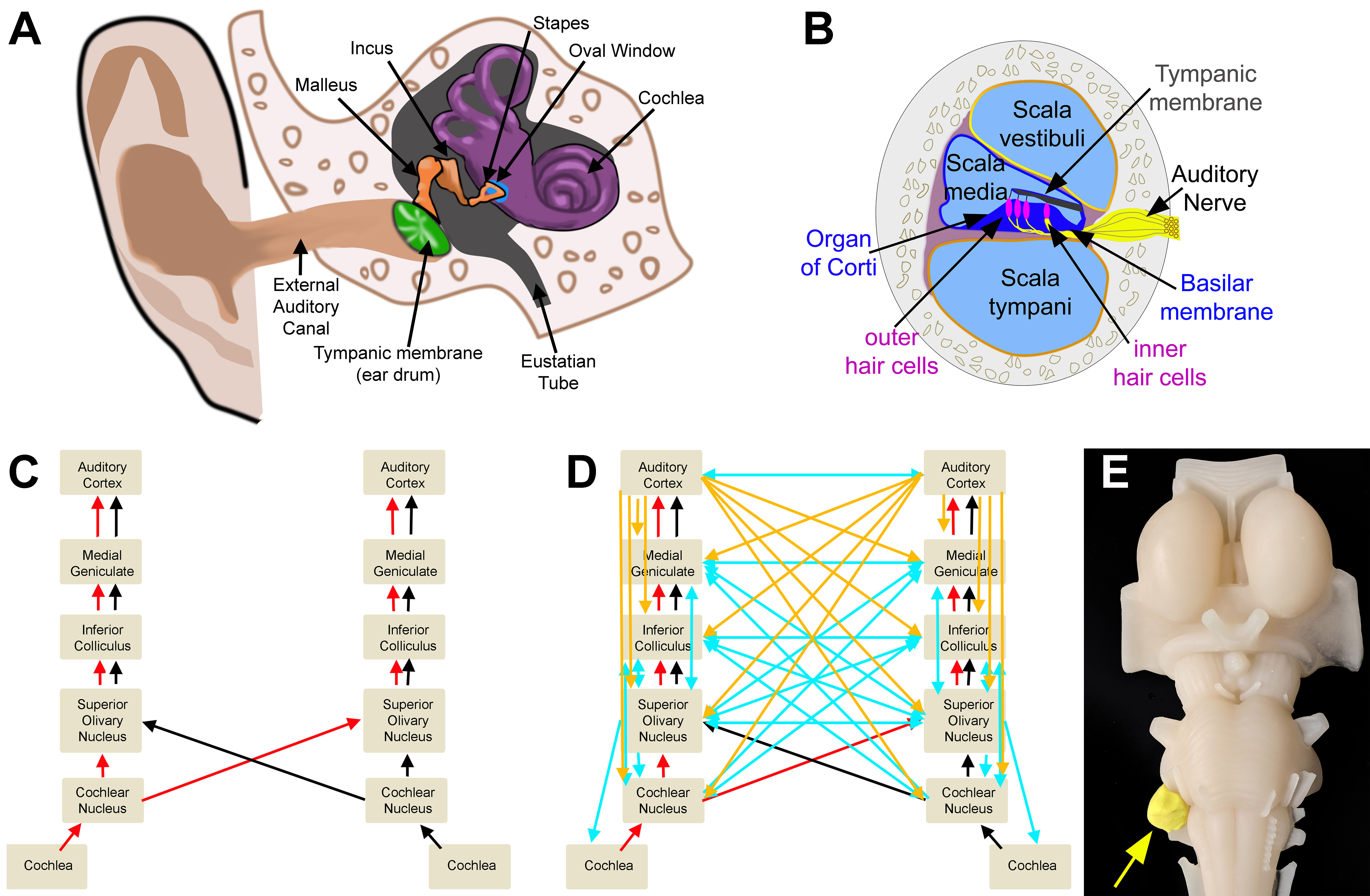
Auditory Circuits. A. Outer, middle, and inner ear. B. Cross section of the cochlea. C. Ascending auditory pathway. D. Ascending (red/black), descending (cortical: orange; brain-stem: blue), and crossed (blue) auditory circuits. E. Model representation of an acoustic neuroma.
All imaging was created by Diana Peterson for the current manuscript.