Histology, Bladder
- Article Author:
- Srinivasa Rao Bolla
- Article Author:
- Nkiruka Odeluga
- Article Editor:
- Raghu Jetti
- Updated:
- 4/15/2020 8:49:34 PM
- For CME on this topic:
- Histology, Bladder CME
- PubMed Link:
- Histology, Bladder
Introduction
The urine formed by the nephrons of the kidneys is transported to the urinary bladder for storage before it gets expelled through the urethra. The urinary bladder is a sac that serves as a reservoir of the urine. It is located in the extraperitoneal space of the pelvis behind the pubic bones and extends into the abdomen when filled with urine. The bladder divideS into two main parts, each with its own features: the upper part, above the ureteric orifices, is composed of the apex and body while the lower part is composed of the fundus, trigone, and neck. The capacity of the bladder is about 500 mL in healthy individuals.[1] As the bladder fills, it stretches, simulating afferent signals. Efferent signals result in contraction of the bladder musculature and relaxation of the urethral sphincter, respectively. In addition to mechanoreceptors, various psychological factors like stress, sense of acceptable surroundings, and emotional status, play a crucial role in the timing and setting of micturition.[2] In this section, we describe the detailed microscopic structure of the urinary bladder wall.
Structure
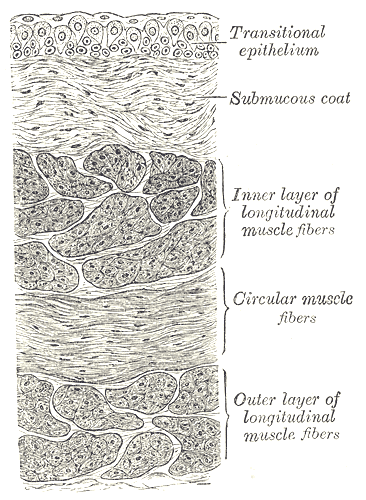
The microscopic structure of the urinary bladder wall organizes into the following layers from inside out.
- Lining epithelium
- Lamina propria
- Muscularis propria
- Serosa/Adventitia
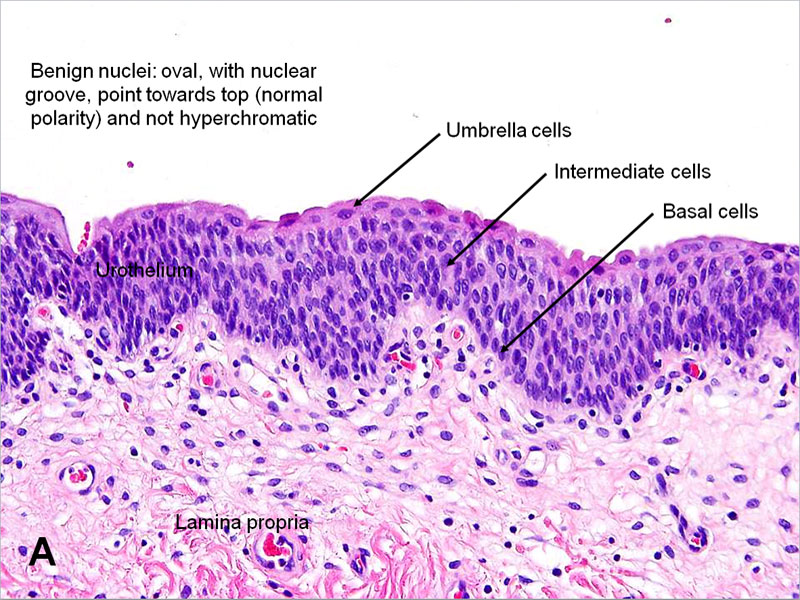
Lining epithelium: The urinary bladder lining is a specialized stratified epithelium, the urothelium. The urothelium is exclusively in urinary structures such as the ureter, urinary bladder, and proximal urethra. The urothelium is composed of three layers[3][4]:
- Apical layer - The innermost layer serves as a barrier between the bladder lumen and the underlying tissue. It is a single layer of umbrella-shaped cells (i.e., umbrella cells) that are frequently binucleated. These apical umbrella cells of the urothelium form an impermeable barrier; tight junctions between the cells decrease paracellular flux while a glycoprotein layer of uroplakin forms a superficial plaque that covers the umbrella cells.
- Intermediate layer - Formed from two to three layers of polygonal cells
- Basal layer - Formed from two to three layers of small cuboidal cells
In a relaxed urinary bladder, the urothelium is five to seven layers thick. When the urinary bladder fills with urine, the bladder wall stretches to accommodate the increased volume. In the distended bladder, the urothelium reorganizes to two or three layers without any structural damage. Due to this transitional ability of the urothelium, it is also known as transitional epithelium.
Lamina Propria: This is the suburothelial layer separating the urothelium and underlying muscularis propria (detrusor muscle). It is separated from the overlying urothelium by a basement membrane. Its composition is an extracellular matrix with elastic fibers, capillaries, lymphatics, immune cells, afferent and efferent nerve endings, fibroblasts, myofibroblasts, adipocytes, interstitial cells of Cajal or telocytes, and indistinct smooth muscle layer, and the muscularis mucosae.
The interstitial cells of Cajal are within the lamina propria; they form a syncytium with the smooth muscle cells and nerve endings. The interstitial cells of Cajal are said to function as pacemaker cells in the gut, urethra, and prostate. In the bladder, they appear to act as nerve signal transducers to the smooth muscle cells of the bladder.[5]
Muscularis propria: Also known as the detrusor muscle, it consists of three layers: inner longitudinal, middle circular, and outer longitudinal. These layers are well defined around the neck of the urinary bladder; however, in the rest of the bladder wall, they run randomly, without orientation. The body of the bladder has a higher smooth muscle content compared with the trigone, reflecting a well-developed network of myofibroblasts of lamina propria and muscularis mucosae.[6]
Serosa: This thin connective tissue layer covers the bladder dome and is continuous with the peritoneal layer of the abdominal wall. It also contains blood vessels of various sizes.
Adventitia: This loose connective tissue layer serves as the bladder’s outer layer in areas of the bladder where there is no serosa.
Function
The urothelium transforms from five to seven cell layers thick in a relaxed state to two or three cell layers thick in a distended bladder. This functional modification of the epithelium does not cause any damage - begetting the name "transitional epithelium" – and allows for the storage of urine. In its superficial layer, umbrella-shaped cells are connected by tight junctions and covered by uroplakin, thus making it a barrier preventing injury to the tissue below.[7] The lamina propria, along with the urothelium, are sensory regions regulating the afferent limb of the micturition reflex.[8] The lamina propria acts as a "functional center" of the bladder because of the presence of many specialized cells. It serves as a capacitance layer of the bladder that determines its compliance.[9] The detrusor muscle of the muscularis propria is under autonomic nervous control. Sympathetic stimulation relaxes the detrusor muscle and contracts the urethral sphincter to allow filling of urine while parasympathetic stimulation contracts the detrusor and relaxes the urethral sphincter to allow micturition.
Tissue Preparation
Tissue biopsy for histopathological examination of the bladder is performed via cystoscopy or from radical cystectomy samples. Diagnosticians can use image-guided percutaneous bladder biopsy if there are contraindications to cystoscopy is contraindicated.[10] Formalin-fixed tissue embedded in paraffin wax or frozen sections is useful for routine hematoxylin and eosin histopathology of the urinary bladder.
Histochemistry and Cytochemistry
The umbrella-shaped top layer of cells is CK20+, and the basal cells are CD44+. In carcinoma in situ, all cells are CK20+, p53+, and show a greater Ki-67 proliferation index.[11]
Microscopy Light
Under the light microscope, in a histological section of the bladder wall, the urothelium, lamina propria, muscularis propria, and serosa may be seen. The most superficial layer of the urothelium is composed of dome-shaped umbrella cells whose shape becomes flat in a distended bladder. The cells of this layer are often multinucleated. Below the umbrella cells, the urothelium contains the multi-cell-layered intermediate and a single-cell layer of basal cells contacting its basement membrane. The cells of the intermediate layer are uninucleated; the number of layers in the intermediate layer depends on the stage of distension of the urinary bladder.[7] The basal layer contains mononucleated, cuboidal cells with mitotic capability. Even though the rate of turnover is gradual, this layer demonstrates great regenerative capacity. The lamina propria is a suburothelial layer, the contents of which are described in the “Structure” section above.
Microscopy Electron
Umbrella cells demonstrate a characteristic ultrastructural feature - plaques or asymmetrical unit membrane - which are thick, focal areas of the cell membrane that are associated with actin filaments. Actin filaments extend from the inner surface of the plaques to the cytoplasm of umbrella cells. In the non-distended bladder, the superficial cells appear to fold inwards; these folded plaques seemingly form membrane-bound, cytoplasmic fusiform vesicles. When the bladder distends during filling, these vesicles unfold to become part of a smooth surface as the cells flatten out.
Pathophysiology
Urge incontinence, a form of urinary incontinence, is characterized by urinary urgency. Etiologies include neurogenic, myogenic, or idiopathic causes. Disruption or damage of autonomic nerves, nerve signaling, or bladder cells may result in conditions like overactive bladder or bladder pain syndrome.[2]
Clinical Significance
Clinicians use cystoscopy to examine the urinary bladder mucosa for pathology, which may be collected via biopsy sampling. Benign lesions include von Brunn’s nests. These islands or nests of the urothelium are separated from the luminal surface and consequently found within the lamina propria. von Brunn’s nests of benign urothelium may undergo degeneration to form cysts (cystitis cystica). Conversely, malignant bladder tumors are graded based on their depth of invasion into the bladder wall.
(Click Image to Enlarge)