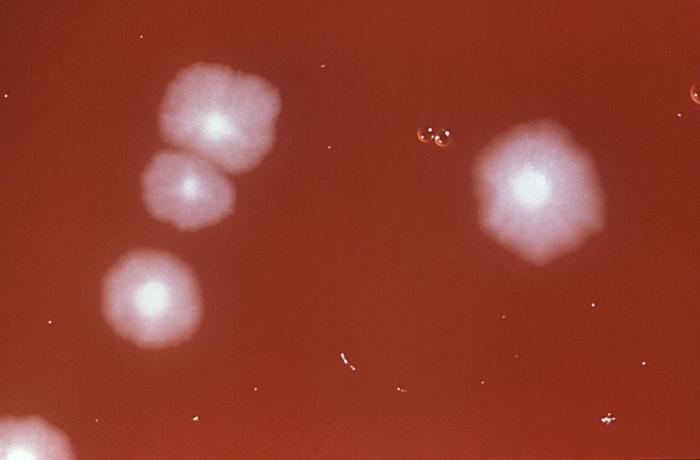
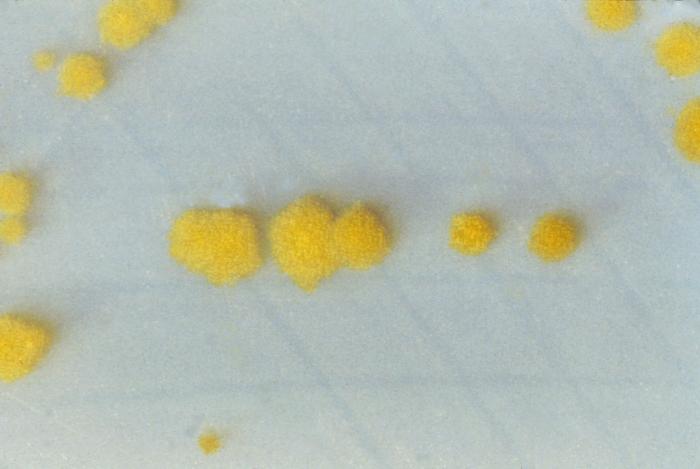
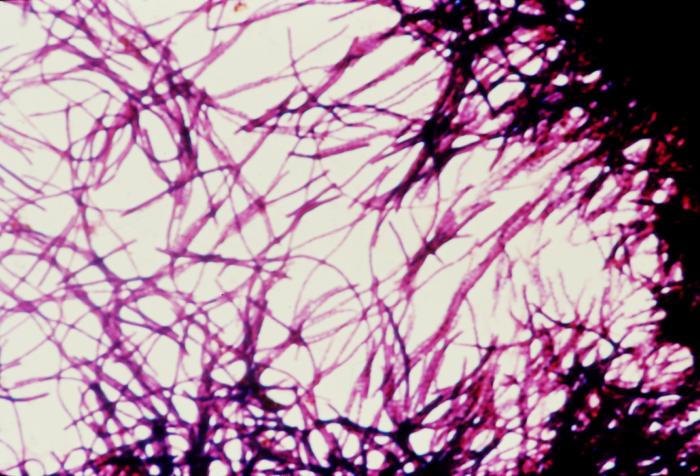
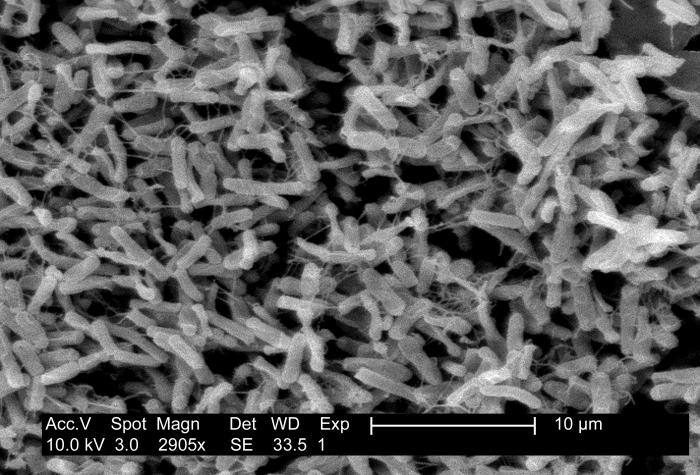
Clostridium Difficile
- Article Author:
- Pradeep Kumar Mada
- Article Editor:
- Mohammed Alam
- Updated:
- 8/8/2020 9:03:29 PM
- For CME on this topic:
- Clostridium Difficile CME
- PubMed Link:
- Clostridium Difficile
Introduction
Clostridium difficile is a gram-positive bacterium that is the cause most implicated in antibiotic-associated diarrhea. The emergence of a newer hypervirulent strain North American pulsed-field gel electrophoresis type 1 (NAP1) has been attributed to the increase in incidence and severity of C. difficile infections (CDI) over the last decade.[1]
Etiology
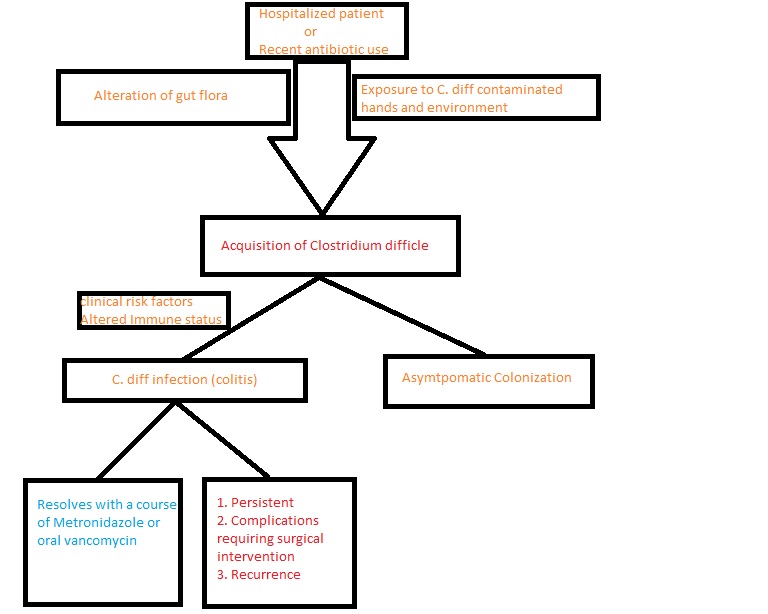
C. difficile is a gram-positive drumstick-shaped bacillus and a spore-forming obligate anaerobe that produces toxins. The organism is commonly found in water, air, human and animal feces, hospital surfaces, and soil. The optimum temperature for organism growth is about the 37-degree Celsius. The primary mode of the disease transmission is the fecal-oral route. The organism produces two types of toxins which are major virulent factors: toxin A and toxin B. While a majority of pathogenic strains associated with C. difficile infection produce both toxin A and toxin B, strains that produce only toxin B have been reported globally.[2]
Antibiotic use remains the leading risk factor for C. difficile infection. Several classes of antibiotics such as penicillins, cephalosporins, fluoroquinolone, and clindamycin are implicated in the disease's cause. Apart from antibiotic usage, other risk factors involved in the causation include advanced age, chemotherapy, use of proton pump inhibitors, chronic renal disease, chronic liver disease, and malnutrition.[3][4]
Epidemiology
As per the recent estimates by the Center for Disease Control (CDC), C. difficile infects approximately half a million Americans each year. Among those infected with C. difficile, about 29,000 patients had fatal outcomes within a month of the initial diagnosis, and 15,000 of these deaths could be directly attributed to the C. difficile infection. Contrary to the popular belief that C. difficile is typically a hospital-acquired infection, recent studies have revealed that approximately 41% of the infections caused by C. difficile are community acquired.[5] [6]Studies have showed a shifting trend in the most commonly affected patient populations with an increasing incidence of C. difficile infection among younger patients and patients without prior exposure to antibiotics. [7]Approximately 83,000 of the patients with C. difficile had at least one recurrence, and 29,000 died of the initial diagnosis within 30 days. Antibiotic misuse increases patient risk for C. difficile infections. More than half of the patients hospitalized will get an antibiotic during their hospitalization, and 30% to 50% of antibiotics prescribed are unnecessary or incorrect. According to CDC estimates, up to $3.8 billion in medical costs could be saved over 5 years.
Pathophysiology
C. difficile colonizes in the large intestine of humans. Healthy adults with adequate immune response become asymptomatic carriers of the disease. Neonates also have a high rate of asymptomatic carrier rate, owing to lack of intestinal receptors for C. difficile.[8] Use of antibiotics alters the microbial flora of the large intestine, rendering it susceptible to infection by C. difficile, and the transmission of the disease occurs through the fecal-oral route. Diarrhea and colitis, the hallmark features of C. difficile infection, are caused by clostridial glycosylation exotoxins, toxin A (TcdA) which is enterotoxin, and toxin B (TcdB) which is cytotoxic. [9]A few pathological strains produce an additional toxin known as a binary toxin, the role of which is not elucidated. [10]Toxin A has a carbohydrate receptor binding site that facilitates the intracellular transport of both toxin A, and toxin B.[11] After becoming intracellular, both the toxins cause inactivation of pathways mediated by the Rho family of proteins, resulting in damage of colonocytes, disruption of intercellular tight junctions, and colitis. [12]Toxin-A causes direct activation of neutrophils while both toxin A and toxin B are involved in the chemotaxis of neutrophils.[13]
History and Physical
Clinical manifestations of the infection caused by C. difficile vary from an asymptomatic carrier to severe disease-causing, toxic megacolon, depending on a wide range of pathogen and host factors. Watery diarrhea with mucus or occult blood, anorexia, nausea, vomiting, low-grade fever, and lower abdominal pain are the symptoms commonly associated with diarrhea and colitis caused by C. difficile.[14] In certain instances, patients develop fulminant colitis characterized by diarrhea, diffuse abdominal pain, abdominal distension, hypovolemia which could lead to sepsis, toxic megacolon, and perforated bowel with peritonitis.[15] Us reported rare presentations such as protein-losing enteropathy and extra-colonic manifestations like appendicitis, reactive arthritis, and in the literature.[16][17] Recurrent infection caused by C. difficile is described as a complete resolution of the symptoms when treated and they stop occurrence of the symptoms.[18]
Evaluation
Patients with new-onset 3 or more loose or unformed stools in 24 hours with no obvious other etiology should be checked for testing for C. difficile infection. Stool examination for C. difficile toxins or toxigenic C. difficile bacillus is the commonly used diagnostic test used to diagnose C. difficile infection. Several techniques such as polymerase chain reaction (PCR), enzyme immune assays for C. difficile toxin A, toxin B, and glutamate dehydrogenase (GDH), cell culture cytotoxic assay, and selective anaerobic culture can be used in making the diagnosis.[19] Studies showed that diagnostic approaches that use PCR either as a single step diagnostic approach or as a part of a multistep approach have the highest rates of sensitivity and specificity regarding diagnosing C. difficile. [14]Additional diagnostic tests such as radiographic imaging of abdomen and pelvis or lower gastrointestinal endoscopy are used in certain instances when patients develop fulminant colitis or alternative diagnoses are suspected. Infectious Diseases Society of America (IDSA) recommends a stool toxin test as part of a multi-step algorithm (GDH plus toxin, GDH plus toxin, arbitrated by nucleic acid amplification test, or NAAT plus toxin). It is not recommended to repeat testing within 7 days during the same episode or perform the test in asymptomatic patients.
Treatment / Management
The management of C. difficile infection includes a multi-step approach of discontinuing the usage of inciting antibiotics, isolating the patient, and administering the antibiotic based on the severity of the infection. The antibiotics usually used in the treatment of C. difficile infection are vancomycin or fidaxomicin. Asymptomatic patients despite having a positive stool toxin test do not require treatment. Intravenous metronidazole can be used in a patient who suffers from ileus where the delivery of orally administered antibiotics will be delayed. However, vancomycin cannot be administered intravenously because it cannot be secreted into the colon.
As per new IDSA guidelines, an initial non-severe C. difficile infection episode in adults can be treated with vancomycin 125 mg orally 4 times/day or fidaxomicin 200 mg twice a day for 10 days.
Severe C. difficile infection is defined as a white blood cell count greater than 15,000 cells/microL, serum albumin less than 3 g/dL, and a serum creatinine level greater than 1.5 times the premorbid level. A severe initial episode can be treated with the same dosage of vancomycin or fidaxomicin as in initial non-severe C. difficile infection.
For an initial fulminant episode with hypotension, shock, ileus, or megacolon, the treatment is vancomycin 500 mg, 4 times/ day orally or by NG tube. If ileus is present, rectal vancomycin enema (500 mg in 100 ml normal saline per rectum 4 times/day) can be used. Intravenous metronidazole 500 mg, 3 times daily can be added with oral or rectal vancomycin. Surgical intervention such as subtotal colectomy with preservation of the rectum may be required in certain patients who develop fulminant colitis leading to complications such as toxic megacolon, intestinal perforation, and necrotizing colitis.[20]
The first recurrence of C. difficile infection can be treated with a prolonged taper and pulsed vancomycin regimen which is 125 mg, 4 times per day for 10 to 14 days, 2 times per day for 7 days, one time a day for 7 days, and lastly, once every 2 to 3 days for 2 to 8 weeks or fidaxomicin 200 mg, 2 times per day for 10 days if vancomycin was used for the initial episode.
A second or further recurrence can be treated with a taper and pulsed vancomycin regimen as mentioned for the first recurrence, vancomycin 125 mg orally, 4 times per day for 10 days followed by rifaximin 400 mg orally, 3 times per day for 20 days, fidaxomicin 200 mg, 2 times per day for 10 days, OR fecal transplantation.
Bezlotoxumab, a newer human monoclonal antibody against C. difficile toxin, has been recently approved for the management of recurrent infections by C. difficile and a clinical study performed to assess the efficacy revealed positive outcomes. [21]Fecal transplantation, the process through which feces from a healthy donor into the intestinal tract of a person with disrupted microbial balance, has reported an 80% to 90% success rate in reducing the recurrence of C. difficile infections.[22]
Differential Diagnosis
- Crohn’s disease
- Diverticulitis
- Irritable Bowel Syndrome (IBS)
- Malabsorption
- Peritonitis
- Salmonella infections
- Shigellosis
- Ulcerative colitis
- Vibrio infections
- Viral gastroenteritis
Pearls and Other Issues
Prevention
Effective prevention of C. difficile infection includes several generalized strategies and certain targeted strategies. General strategies such as early detection of the disease, placing the patient under isolation with a dedicated toilet and contact precautions, promoting hygiene measures such as improved hand hygiene, and environmental cleaning are effective measures in preventing infections from C. difficile infections. These general strategies are important not only in the hospital setting but also at the community level. Targeted strategies include the use of antibiotics which are least associated with the causation of C. difficile infection. [23]
Ongoing Research
Drugs such as ridinilazole are being studied in the effective treatment of Clostridium difficile infection. Ribaxamasean oral beta-lactamase can maintain gut bacteria and is being studied for the prevention of C. difficile infection and antibiotic-associated diarrhea. Microbiome therapies are also being investigated as an alternative approach to preventing antibiotic-associated diarrhea. Pharmaceutical companies are developing a vaccine to prevent C. difficile infection.
Enhancing Healthcare Team Outcomes
Hand hygiene should be performed with soap and water instead of alcohol-based products when caring for a patient with C. difficile infection because of increased efficacy of spore removal with soap and water.
To prevent C. difficile infections use infection control recommendations and more prudent antibiotic use. It is recommended to continue contact precautions for at least 2 days after diarrhea has resolved and continue contact precautions until discharge if infection rates remain high despite using standard infection control measures.
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)