Diabetic Perioperative Management
- Article Author:
- Prerna Dogra
- Article Editor:
- Ishwarlal Jialal
- Updated:
- 6/3/2020 4:36:38 PM
- For CME on this topic:
- Diabetic Perioperative Management CME
- PubMed Link:
- Diabetic Perioperative Management
Introduction
In both diabetic and non-diabetic populations, hyperglycemia in the perioperative period is an independent marker of poor surgical outcomes (delayed wound healing, increased rate of infection, prolonged hospital stay, higher postoperative mortality).[1][2][3][4] Hyperglycemia (greater than 140 mg/dl) is a frequent occurrence with a prevalence of 20 to 40% in the general surgery and 80 to 90% in the cardiac surgery population.[2][4][5][6]
The stress of surgery, anesthesia, and illness increases secretion of counter-regulatory hormones (cortisol, glucagon, growth hormone, catecholamines), which in turn causes decreased insulin secretion, increased insulin resistance, decreased peripheral utilization of glucose, increased lipolysis and proteolysis. As a consequence, gluconeogenesis and glycogenolysis increase, which subsequently results in worsening hyperglycemia termed as stress hyperglycemia. Uncontrolled hyperglycemia instigates osmotic diuresis (causing fluid and electrolyte imbalance), ketogenesis and increased generation of pro-inflammatory cytokines with resultant mitochondrial injury, endothelial dysfunction and immune deregulation.[7][8] Hence, achieving good glucose control during the perioperative period is associated with beneficial post-surgical outcomes.[4][5]
The severity of hyperglycemia also depends on the type of anesthesia and surgery, with increased glucose elevations seen in cases of general anesthesia or thoracic/abdominal surgeries as opposed to epidural/local anesthesia or peripheral/laparoscopic surgeries, respectively.[9][10][11]
Issues of Concern
PERIOPERATIVE MANAGEMENT
The perioperative period divides into three phases-
- Preoperative
- Intraoperative
- Postoperative
1. Preoperative
A) History
It is essential to obtain a detailed account regarding
- Diabetes mellitus (DM) – the type of diabetes, management (lifestyle modifications and medications), current glycemic control, related complications (nephropathy, neuropathy, retinopathy, cardiovascular disease), and susceptibility to hypoglycemia including hypoglycemic unawareness. If on antidiabetic drugs, it is imperative to know the details of the regimen and about medication adherence.
- Surgery - ambulatory/inpatient, elective/time-sensitive/emergent, the anticipated duration of surgery and fasting.
B) Glycated Hemoglobin A1c
A preoperative hemoglobin A1c (HbA1c) should be checked, if not tested in the preceding three months. Multiple studies have looked at the association of HbA1c and surgical outcomes, and based on existing literature, it is controversial whether elevated HbA1c is linked to poor postoperative outcomes or is just a marker of poor perioperative glucose control.[12][13][14][15][16] Besides, there is no evidence proving better outcomes by deferring the surgery for better glycemic control. Although there are no validated HbA1c cut-off values, it may be plausible to postpone an elective surgery if HbA1c is higher than 10%. Procedures that are of emergent or time-sensitive nature should not be delayed to achieve a target HbA1c; instead, the focus should be on optimizing perioperative glucose control. Nevertheless, it is recommended to obtain a preoperative HbA1c to assess glycemic control and recognize patients with undiagnosed diabetes.
C) Oral Antihyperglycemic & Non-Insulin Injectable
There is concern regarding the safety and efficacy of oral antihyperglycemic and non-insulin injectable in the perioperative or hospital settings. Metformin can lead to the development of lactic acidosis in cases of renal dysfunction or with use of intravenous contrast, sulfonylureas and other insulin secretagogues risk hypoglycemia, sodium-glucose cotransporter-2 (SGLT-2) inhibitors carry the risk of euglycemic ketoacidosis in fasting, or acutely ill patients, glucagon-like-peptide-1 receptor (GLP-1) agonists can worsen nausea and vomiting by delaying gastric emptying. Furthermore, the delayed onset and prolonged duration of action make it challenging to titrate these medications to achieve optimal glycemic control over a short period. Currently, the recommendations are for holding these medications on the day of surgery except for SGLT-2 inhibitors [17][18], which should be held minimal 24-hours before surgery. In cases of emergent surgery or illness, these medications should be stopped immediately.
Recent evidence from randomized controlled trials such as the SITA-HOSPITAL trial demonstrated that dipeptidyl peptidase-4 (DPP-4) inhibitors are both safe and efficacious in medical and surgical patients with mild to moderate hyperglycemia; however some such as saxagliptin predispose to heart failure.[19][20][21] However, recent guidelines published by ADA, do not recommend the use of DPP-4 inhibitors in the inpatient setting. There is also an emerging interest regarding the use of GLP-1 agonists in the hospital setting, and multiple large RCTs are currently underway.[22]
D) Insulin Therapy
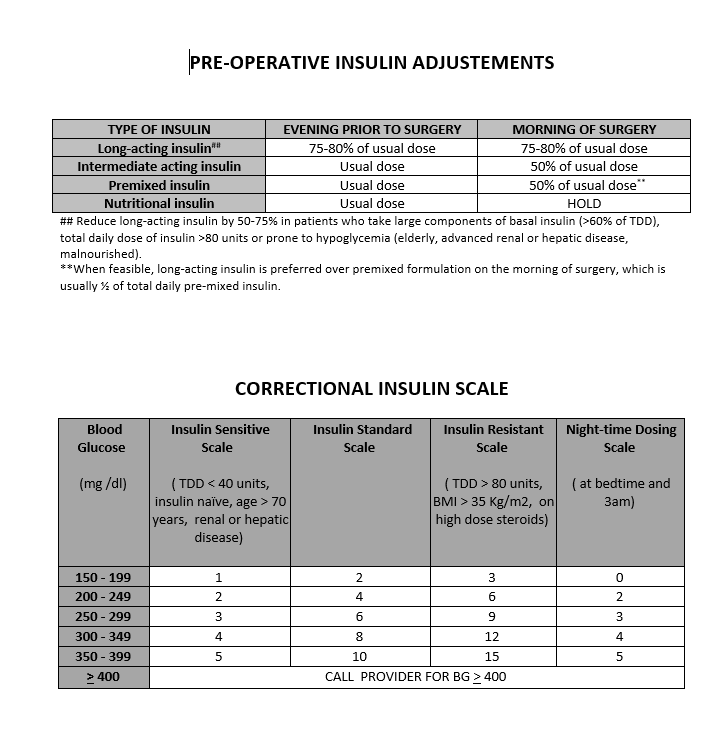
Patients who are on home insulin therapy should reduce the dose of long-acting basal insulin (glargine, detemir) by 20-25% the evening before surgery.[23] If they routinely take basal insulin only in the morning, then the reduced dose should instead be administered on the morning of surgery. Patients who are on twice daily glargine or detemir should reduce the dose by 20 to 25% in the evening prior to as well as the morning of surgery. However, in patients who take high doses of basal insulin (>60% of total daily insulin) or total daily insulin dose is greater than 80 units or are at high risk of hypoglycemia (elderly, renal or hepatic insufficiency, prior hypoglycemic episodes); basal insulin dose should be reduced by 50 to 75% to minimize hypoglycemia risk. For the ultra-long-acting insulin, owing to their long half-life, dose reductions should be made three days before surgery with the help of an endocrinologist or diabetes care team. In cases of intermediate-acting insulin such as neutral protamine hagedorn (NPH), the usual dose is administered the evening prior and reduced by 50% on the morning of surgery. Patients who are on premixed insulin (NPH/Regular 70/30, aspart protamine/aspart 75/25, etc.), should preferably receive long-acting insulin the evening prior instead of their premixed formulation. However, this may not be feasible in a lot of these patients. In such scenarios, the premixed insulin is reduced by 50% on the morning of surgery, followed by the initiation of dextrose-containing intravenous solutions. Alternatively, these patients can be asked to skip the morning dose and arrive early to the preoperative area where they can receive a long-acting formulation.[Image]
During the fasting state, nutritional (or prandial) insulin is held, and subcutaneous correctional insulin initiated with blood glucose (BG) monitoring every 4 to 6 hours. Most institutions have standardized correctional insulin scales based on different insulin sensitivities.[24] [Image]
In critically ill patients, continuous intravenous infusion (CII) using regular insulin is the preferred regimen. In the setting of hemodynamic instability/hypothermia/peripheral vasoconstriction, subcutaneous insulin is absorbed poorly, and intravenous insulin is preferable due to more predictable pharmacokinetics. Furthermore, intravenous insulin allows for easy dose titration due to a shorter duration of action (10 to 15 minutes) and omits the need for multiple injections. The use of CII should always be governed by a validated institutional protocol that includes a standardized approach for infusion preparation, initiation, titration, and monitoring.[25]
Diabetic patients should preferably be scheduled for surgery early in the day. It is recommended to check the blood glucose in the preoperative area. Hypoglycemia (BG less than 70 mg/dl) treatment is with glucose tablets/gels or intravenous dextrose solutions. In cases of severe hyperglycemia (BG greater than 250 mg/dl) or metabolic decompensation (diabetic ketoacidosis or hyperglycemic hyperosmolar syndrome), it is prudent to postpone surgery by a couple of hours for better glycemic control.
2. Intraoperative
Hyperglycemia (over 180 mg/dl), in surgeries of shorter duration (<less than 4 hours) with expected hemodynamic stability and minimal fluid shift, can be managed with 2-hourly subcutaneous correctional insulin (preferably rapid-acting insulin) and BG checks. In surgeries that may involve hemodynamic fluctuations, massive fluid shifts, or last longer than 4 hours duration, BG greater than 180 mg/dl should be managed with intravenous insulin infusion, and BG monitored every 1 to 2 hours.
3. Postoperative
In the post-anesthesia care unit (PACU), it is imperative to review the intraoperative hyperglycemia management and continue close glucose monitoring with either intravenous or subcutaneous insulin.
A) Ambulatory
After recovery in the PACU, ambulatory surgery patients who are stable and tolerating oral intake can be discharged home on the previous antihyperglycemic regimen.
B) Non-critically Ill
Non-critically ill patients who require hospitalization are admitted from PACU to the surgical/medical ward on subcutaneous (SC) insulin. In the case of poor or no oral intake, basal plus correctional insulin is preferred [26]. While in a patient with regular oral intake, the insulin regimen should consist of basal, nutritional, and correctional components.
- Basal insulin: Controls hyperglycemia when a patient is not eating (at night, in between meals or when fasting) and can be given as long-acting insulin (glargine or detemir) once or twice daily.
- Nutritional insulin: Also referred to as meal-time or prandial insulin, helps control hyperglycemia related to carbohydrate intake (meals, enteral, or parenteral nutrition), with either rapid-acting insulin (lispro, aspart or glulisine) or short-acting insulin (regular).
- Correctional insulin: Is used to counteract hyperglycemia that is above the goal, with either rapid-acting or short-acting insulin. When correctional insulin is given in addition to nutritional insulin, then the same formulation is combined into one single dose.
The insulin regimen can be dosed based on weight or pre-hospitalization regimen. Patients on home insulin regimen with good glycemic control should have their basal insulin reduced by 20 to 25% if the oral intake is inadequate. For weight-based dosing; in an average patient, the starting total daily dose (TDD) of insulin is 0.4 to 0.5 U/Kg/day; in insulin-sensitive patients (type 1 DM, insulin naïve, elderly, malnourished, renal/hepatic insufficiency, frequent hypoglycemia) starting dose should be reduced to 0.2 to 0.3 U/Kg/day and in insulin-resistant (obese, on high-dose steroids) starting dose should be increased to 0.6 to 0.7 U/Kg/day. If a patient has features belonging to both insulin sensitive and resistant categories, then it is safer to dose as insulin-sensitive. Once the TDD is determined, half of TDD is administered as basal insulin, and 1/6 of TDD will be administered as nutritional insulin with each of the three meals.[27][28] When eating, BG is generally monitored four times a day (before meals and at bedtime), and correctional insulin administered accordingly. The patient who is receiving nothing by mouth should have BG monitored every 6 hours for correction with regular insulin or every 4 hours for correction with rapid-acting insulin.
C) Critically Ill
Critically ill patients should be managed in a medical or surgical intensive care unit with continuous insulin infusion (CII) with regular insulin, with BG monitoring every 1 to 2 hours, as dictated by institutional protocol. The transition from CII to long or intermediate-acting SC insulin is done once these patients are hemodynamically stable with no vasopressor requirement, have optimal glycemic control with minimal variability, and a steady infusion rate in the past 6 to 8 hours. Due to the extremely short half-life of intravenous insulin and delayed onset of action of long/intermediate-acting insulin, it is essential to overlap IV and SC insulin by 2 to 3 hours. Premature discontinuation of IV insulin creates a hiatus in the basal insulin supply, which risks rebound hyperglycemia or metabolic decompensation (especially in type 1 diabetes).
Subcutaneous basal insulin at the time of transition is dosed based on either (a) the rate of insulin infusion, or (b) weight, or (c) home insulin dose. When using the rate of infusion to calculate the basal insulin dose, the average rate of infusion over the last 6 to 8 hours gets extrapolated to 24 hours. Seventy to eighty percent of this extrapolated dose represents TDD. In a patient with minimal or no caloric intake, 100% of the calculated TDD is administered as basal. In contrast, in patients with optimal caloric intake, 50% is given as basal, and 50% as nutritional insulin. For the weight-based method, TDD is calculated similarly to non-critically ill patients, half of which is a basal dose and the other half as nutritional insulin. In patients with good glycemic control on home insulin therapy, 70 to 80% of the home basal insulin dose can be administered at the time of transition. After the transition, similar to non-critically ill patients, hyperglycemia is managed with correctional insulin every 4 to 6 hours in a fasting patient and four times a day (before to meals and at bedtime) in a patient who is eating.[29][30]
Due to unpredictable glycemic fluctuations, the sole use of correctional insulin is not recommended.[31][32] Additionally, premixed insulin regimens should be avoided in the perioperative setting due to the increased risk of hypoglycemia.[33] The use of oral or non-insulin antihyperglycemic is an area of active research and currently not recommended for glucose management in these patients.[34]
The insulin dosing explained in this article is just a starting point, and nearly all patients will require ongoing adjustments to their insulin regimen based on blood glucose, nutritional intake, and changes in clinical status. Moreover, glucose trends are more important than individual BG readings when making adjustments to the regimen.
Clinical Significance
In critically ill patients, multiple randomized controlled trials (RCTs) like NICE-SUGAR study have compared conventional (less than 180 mg/dl) versus intensive (81 to 108 mg/dl) glucose control with results remarkable for a higher incidence of severe hypoglycemia and increased mortality in patients subjected to intensive glucose control.[5][35][36][37] However, due to the lack of RCTs in non-critically ill patients, most of the data is extrapolated from studies conducted on the critically ill.
Multiple societies have put forth guidelines for optimal glucose management in the perioperative period. For patients undergoing ambulatory surgery, the Society for Ambulatory Anesthesia recommends intraoperative blood glucose (BG) levels less than 180 mg/dl. In critically ill patients, the Society of Critical Care Medicine recommends initiating insulin therapy for BG higher than 150 mg/dl, the American College of Physicians advises against the use of intensive insulin therapy with a BG target of 140 to 200 mg/dl. The Society of Thoracic Surgeons advocates intra-operative blood glucose less than 180 mg/dl and lower than 110 mg/dl in the pre-meal or fasting state. In the non-critically ill hospitalized patients, Endocrine Society recommends pre-meal glucose targets less than 140 mg/dl and random glucose levels lower than 180 mg/dl while the Joint British Diabetes Societies propose blood glucose levels of 108 to 180 mg/dl in most patients with an acceptable range between 72 to 216 mg/dl. The Endocrine Society also outlines higher target glucose of under 200 mg/dl is acceptable in non-critically ill hospitalized patients with a terminal illness and with limited life expectancy or at high risk for hypoglycemia. American Diabetes Association (ADA) recommends a target glucose range of 80 to 180 mg/dl in the perioperative period and 140 to 180 mg/dl for the majority of critically ill and non-critically ill patients.[34].
Although the optimal glycemic target remains unclear, a reasonable goal in the majority of perioperative patients is to maintain blood glucose in the range of 140 to 180 mg/dl with the intent of avoiding both hypoglycemia (under 70 mg/dl) and severe hyperglycemia (over 180 mg/dl).
Other Issues
Type 1 Diabetes Mellitus
Due to minimal to no pancreatic beta-cell function, type 1 diabetics should have a basal-supply of insulin at all times (even if nothing by mouth), either subcutaneously or intravenously. Failure to do so can cause them to decompensate into diabetic ketoacidosis easily.
Subcutaneous Insulin Pumps
In recent years, the use of an insulin pump has increased exponentially, especially in type 1 diabetes. The use of insulin pumps in the hospital setting during the pre-/postoperative period must be guided by clear institutional policies and the patient’s ability to manage the pump.[38][39] Continuation of insulin pump intraoperatively should be restricted to procedures of fewer than 2 hours duration, at the discretion of the anesthesiologist. Insulin pumps provide basal coverage with a continuous subcutaneous infusion of small doses of rapid-acting insulin. Nutritional and correctional bolus coverage is achieved by manually pushing a button to dispense the required amount of rapid-acting insulin. If not feasible to continue the pump, these patients should transition to a subcutaneous basal-bolus regimen. It is recommended to administer the basal insulin at least 2 hours before discontinuation of the insulin pump. This crucial step will prevent any lapse in basal insulin supply and subsequent rebound hyperglycemia or metabolic decompensation. The dose of long-acting basal insulin to be administered is equivalent to the 24-hour basal dose of insulin delivered by the pump. An alternative method is to calculate the basal requirement based on weight considering individual insulin sensitivity.[40]
Enteral Nutrition
Diabetic patients on enteral nutrition should preferably receive formulas containing low carbohydrates, high mono-saturated fatty acids, and subcutaneous insulin regimen should include basal, nutritional, and correctional components. Usually, 30 to 50% of TDD is administered as basal insulin, once or twice daily. The remaining 50 to 70% of TDD is added as nutritional insulin. An alternative method for calculating the dose of nutritional insulin is to administer 1 unit of rapid-acting or regular insulin for every 10 to 15 grams of carbohydrates that will be provided with each bolus feed or in 24 hours with a continuous feed.[34][41][42] In patients receiving continuous tube feeds, nutritional and correctional insulin is administered every 4 hours (with rapid-acting insulin) or every 6 hours (with regular insulin) while those receiving bolus enteral feeds, the nutritional and correctional insulin is given before the bolus feed.
Parenteral Nutrition
In patients receiving total parenteral nutrition (TPN), insulin can be provided either as a separate intravenous infusion or added to the TPN solution. When added to the solution, a starting point is to add 1 unit of regular insulin for every 10 grams of dextrose, and the regimen adjusted every 1 to 2 days based on glycemic trends. Another method is to start a separate intravenous infusion initially. Once BG is within the glycemic goal, 80 to 100% of the total insulin dose provided via infusion is added to the TPN solution. Additionally, BG monitoring with subcutaneous correctional insulin every 4 to 6 hours is used to treat any hyperglycemia above the target range [34][43].
Hypoglycemia
Based on the classification by International Hypoglycemia Study Group – BG less than 70 mg/dl is the hypoglycemia alert value (level 1), BG level less than 54 mg/dl indicates clinically significant hypoglycemia (level 2) and cognitive impairment with no specific BG threshold qualifies as severe hypoglycemia (level 3).[44] Iatrogenic hypoglycemia is the most dangerous adverse effect of antihyperglycemic therapy and is a major limiting factor in optimizing glycemic management in diabetic patients.
Iatrogenic hypoglycemia is a common occurrence in the perioperative setting and correlates to poor patient outcomes and mortality.[37][45] Inappropriately dosed insulin, aggressive glycemic target, insulin administration not aligned with meal-time, insulin stacking, unforeseen changes in caloric intake, poor provider-provider or provider-nurse communication, failure to recognize glycemic trends or intervene are a few factors that risk hypoglycemia.[46] Every institution should implement a hypoglycemia management protocol that entails a plan for both the prevention and treatment of hypoglycemia.[47] Per ADA recommendations, treatment regimen should be reviewed and, if necessary, changed when BG lower than 70 mg/dl is noted, as this usually precedes imminent severe hypoglycemia.
Enhancing Healthcare Team Outcomes
Interprofessional communication and care coordination amongst physicians (surgeons, anesthesiologists, hospitalists, endocrinologists, primary care providers), nurses, pharmacists, nutritionists & diabetes educators play a vital role in the optimal management of diabetes in the perioperative period. Adequate communication helps minimizes adverse events, improves clinical outcomes, and patient satisfaction. It is essential to formulate a structured plan tailored to individual patient needs. Implementation of standardized protocols and computerized algorithms by hospitals is instrumental in reducing errors and providing quality care.[48] The patient and/or family should always receive both written and verbal instructions regarding changes in the medication regimen pre- and postoperatively.
A safe transition from inpatient to outpatient setting is a crucial component of diabetes management. The discharge plan should include medication reconciliation, patient education with a doctor, pharmacist, or nurse with specialty training in diabetes education, an evaluation of socioeconomic issues, and communication with an outpatient provider. Should the pharmacist have concerns after the medication reconciliation, it should be addressed with the team. The diabetic nurse educator should make sure both the patient and family understand the outpatient management plan. Again, if there are concerns over the potential lack of understanding or followup, it should be reported to the interprofessional team and addressed. A follow-up visit with the primary care provider or endocrinologist within one month of discharge is advisable for all patients. An earlier appointment within 1 to 2 weeks is preferred if there has been a change in medications, or glycemic control is not optimal at the time of discharge. Patients should receive prescriptions, drugs, and necessary medical equipment to help bridge care with their outpatient follow-up visits, and a coordinated interprofessional strategy is the best means to achieve this.[34] [Level 5]