Pleural Effusion
- Article Author:
- Rachana Krishna
- Article Editor:
- Mohan Rudrappa
- Updated:
- 10/28/2020 12:14:26 AM
- For CME on this topic:
- Pleural Effusion CME
- PubMed Link:
- Pleural Effusion
Introduction
Pleural effusion is the accumulation of fluid in between the parietal and visceral pleura, called pleural cavity. It can occur by itself or can be the result of surrounding parenchymal disease like infection, malignancy or inflammatory conditions. Pleural effusion is one of the major causes of pulmonary mortality and morbidity. [1][2][3]
All healthy humans have a small amount of pleural fluid that lubricates the space and facilitates normal lung movements during respiration. This delicate balance of fluid is maintained by the oncotic and hydrostatic pressure and the lymphatic drainage; disturbances in any one of these systems can lead to a build-up of pleural fluid.
Etiology
Pleural fluid is classified as a transudate or exudate based on modified Light’s criteria. Pleural fluid is considered an exudative effusion if at least one of the criteria are met. [4][5]
- Pleural fluid protein/serum protein ratio more than 0.5
- Pleural fluid lactate dehydrogenase (LDH)/serum LDH ratio of more than 0.6
- Pleural fluid LDH is more than two-thirds of the upper limits of normal laboratory value for serum LDH.
Common causes of transudates include conditions which alter the hydrostatic or oncotic pressures in the pleural space like congestive left heart failure, nephrotic syndrome, liver cirrhosis, hypoalbuminemia leading to malnutrition and with the initiation of peritoneal dialysis.
Common causes of exudates include pulmonary infections like pneumonia or tuberculosis, malignancy, inflammatory disorders like pancreatitis, lupus, rheumatoid arthritis, post-cardiac injury syndrome, chylothorax (due to lymphatic obstruction), hemothorax (blood in pleural space) and benign asbestos pleural effusion.
Some of the less common causes of pleural effusion are a pulmonary embolism which can be exudate or transudate, drug-induced (e.g., methotrexate, amiodarone, phenytoin, dasatinib, usually exudate), post-radiotherapy (exudate), esophageal rupture (exudate) and ovarian hyperstimulation syndrome (exudate).
Epidemiology
Pleural effusion is the most common disease among all the pleural disease and affects 1.5 million patients per year in the United States. A wide variety of diseases can present with pleural effusions like diseases primarily involving the lung like pneumonia, asbestos exposure, primarily systemic diseases like lupus, rheumatoid arthritis, or maybe the pleural manifestation of diseases which primarily affect other organs like congestive heart failure, pancreatitis, or diseases local to the pleura like pleural infections and mesothelioma.[6]
Pathophysiology
In the normal healthy adult, the pleural cavity has minimal fluid which acts a lubricant for the two pleural surfaces. The amount of pleural fluid is around at 0.1 ml/kg to 0.3 ml/kg and is constantly exchanged. Pleural fluid originates from the vasculature of parietal pleura surfaces and is absorbed back by lymphatics in the dependent diaphragmatic and mediastinal surfaces of parietal pleura. Hydrostatic pressure from the systemic vessels that supply the parietal pleura is thought to drive the interstitial fluid into the pleural space and hence has a lower protein content than serum. Accumulation of excess fluid can occur if there is excessive production or decreased absorption or both overwhelming the normal homeostatic mechanism. If pleural effusion is mainly due to Mechanisms that lead to pleural effusion mainly due to increased hydrostatic pressure are usually transudative, and leading to pleural effusion have altered the balance between hydrostatic and oncotic pressures (usually transudates), increased mesothelial and capillary permeability (usually exudates) or impaired lymphatic drainage.[7][8]
History and Physical
A patient with pleural effusion can be asymptomatic or can present with exertional breathlessness depending on the impairment of thoracic excursion. Patient with active pleural inflammation called pleurisy complains of sharp, severe, localized crescendo/ decrescendo pain with breathing or a cough. When the effusion develops, pain can subside, falsely implying an improvement in condition. Constant pain is also a hallmark of malignant diseases like mesothelioma. Depending on the cause of effusion, the patient can also complain of a cough, fever and systemic symptoms.
The physical examination can be subtle. In large effusion, there will be the fullness of intercostal spaces, and dullness on percussion on that side. Auscultation reveals decreased breath sounds and decreased tactile and vocal fremitus. Egophony is most pronounced at the superior aspect of the effusion.
Pleural rub, often mistaken for coarse crackles can be heard during active pleurisy without any effusion.
As pleural effusion is the result of varied disease, history and physical examination should also be focused on the underlying pulmonary or systemic cause of the effusion. For example, in congestive heart failure (CHF), examine for jugular venous distension, S3, and pedal edema, in cirrhosis leading to hepatic hydrothorax, look for ascites and other stigmata of liver disease.
Evaluation
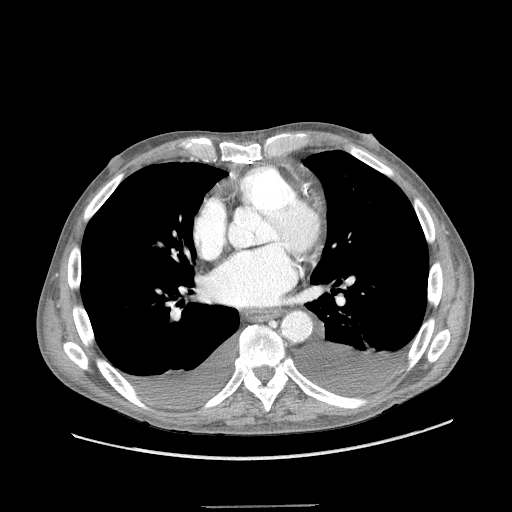
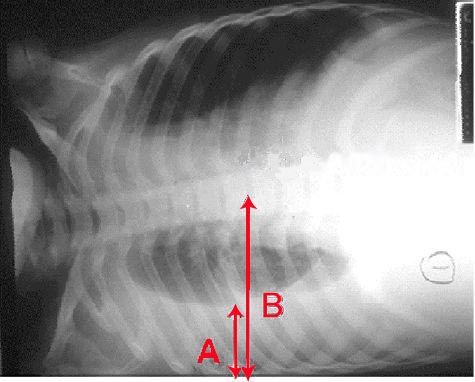
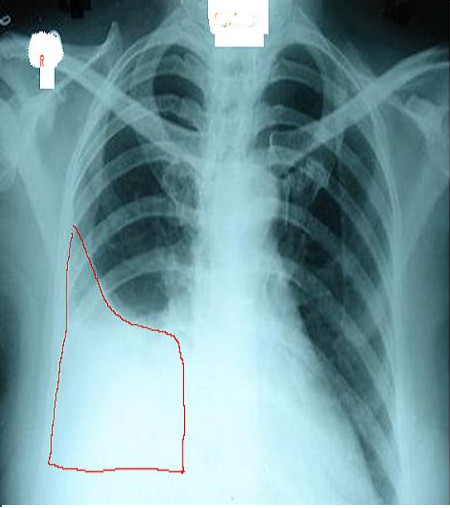
Chest radiographs are useful to confirm the presence of effusion. The findings of effusion vary with amount of effusion. On an upright posteroanterior (PA) view, minimum 200ml of fluid is required to obliterate the costophrenic angle, called the meniscus sign of a pleural effusion. However, in a lateral view, 50 ml of fluid can be diagnosed with this sign. Ultrasound of chest is more sensitive and useful for diagnosis of pleural effusion and also helps in planning thoracentesis. All unilateral effusion in adults needs thoracentesis to determine the cause of pleural fluid. This is also known to improve the patient's symptoms and facilitate recovery.[9][10][11]
Determining whether the fluid is an exudate or transudate narrows the differential. However, Light’s criteria should be interpreted in the clinical context since it misdiagnoses 20% of transudates as exudative. An example would be a patient who has been chronically diuresed for heart failure can increase the pleural fluid protein level and can be classified as an exudate.
Commonly performed tests on the pleural fluid to determine etiology are a measurement of fluid pH, fluid protein, albumin and LDH, fluid glucose, fluid triglyceride, fluid cell count differential, fluid gram stain and culture, and fluid cytology. Exudates are characterized by elevated protein, elevated LDH and decreased glucose. Pleural fluid LDH greater than 1000 U/L may be seen in tuberculosis, lymphoma, and empyema. Low pH (pH less than 7.2) indicates complex pleural effusion in the setting of pneumonia, and almost always requires chest tube insertion for drainage. Other causes for low pH may be an esophageal rupture and rheumatoid arthritis.
Fluid cell counts in transudates show predominantly mesothelial cells. In parapneumonic effusions, lupus pleuritis, and acute pancreatitis, there is neutrophilic predominance in cell counts. Some causes of lymphocyte-predominant effusions include malignancy, lymphoma, tuberculosis, sarcoid, chronic rheumatoid pleural effusion, and malignancy. Eosinophilia in pleural effusion is rare and usually in the presence of air (pneumothorax), blood (hemothorax), a parasitic disease, or drug-induced effusion.
The presence of organisms by gram stain or culture leads to a diagnosis of empyema and necessitates a chest tube for drainage of pus. Cytology is necessary for determining the presence of malignant cells in the pleural fluid. The sensitivity of pleural fluid cytology in the presence of malignant effusion in the first thoracentesis is around 60%, and the yield increases with further attempts, approaching 95% by three samples on different days. However, if a malignant effusion is strongly suspected and cytology is negative, then medical thoracoscopy with pleural biopsy can be performed after two to three thoracenteses to obtain a diagnosis.
Other tests that can be performed on the pleural fluid to determine etiology include adenosine deaminase (ADA) which, when elevated is suspicious for tuberculosis in areas of high prevalence of tuberculosis. In esophageal rupture, the presence of amylase in pleural fluid is diagnostic. In heart failure, an elevated NT-proBNP level may be seen in pleural fluid. The presence of more than 110 mg/dL of triglycerides in the pleural fluid indicates a chylothorax. Pleural fluid is usually straw-colored, and if it is milky white, then a chylothorax should be suspected. Diagnosis of hemothorax can be made if the pleural fluid hematocrit is more than 0.5 times that of serum hematocrit.
The chest x-ray may reveal mediastinal shift to the contralateral chest cavity. There may also be displacement of the trachea towards the ipsilateral side if the bronchus is obstructed. Ct scan is useful to determine the cause like a malignancy.
Treatment / Management
Once the etiology of pleural effusion is determined, management involves addressing the underlying cause. In cases of complex parapneumonic effusions or empyema, (pleural fluid pH less than 7.2 or presence of organisms) chest tube drainage is usually indicated along with antibiotics. Small-bore drains (10 G to 14 G) are equally effective as large-bore drains for this purpose. If patients do not respond to appropriate antibiotics and adequate drainage, then thoracoscopic decortication or debridement may be necessary. Instillation of intrapleural fibrinolytics and DNAse may be used to improve drainage and in those who do not respond to sufficient antibiotic therapy and those who are not candidates for surgical intervention.
If a patient with malignant pleural effusion is not symptomatic, drainage is not always indicated unless an underlying infection is suspected. For malignant pleural effusions that require frequent drainage, options for management are pleurodesis (where the pleural space is obliterated either mechanically or chemically by inducing irritants into the pleural space) and tunneled pleural catheter placement.[12][13]
Chylous effusions are initially managed conservatively but most require surgery.
One should not remove more than 1500 ml of fluid during a single attempt as it can lead to re-expansion pulmonary edema. A chest x-ray is mandatory after performing thoracentesis to determine residual fluid and presence of a pneumothorax.
Differential Diagnosis
- Congestive heart failure
- Injury to the diaphragm
- Diaphragmatic paralysis
- Malignant mesothelioma
- Pneumonia
- Atelectasis
Staging
Current guidelines on the management of Pleural effusions
- Use of bedside ultrasound improves success rates and reduce the risk of pneumothorax during aspiration
- Ultrasound can detect pleural fluid sequestrations
- Always send fluid for biochemistry, culture, and cytology
- Use light's criteria to distinguish exudate from transudate
- Lymphocyte predominant effusions are usually due to heart failure, malignancy, and TB
- Check pH when aspirating pleural effusions
- Do not inject air or local anesthetic into the sample as this may alter the pH of the fluid
- If pH is less than 7.2, drainage of the fluid is recommended
- Malignant effusions can be detected on cytology (40-60%)
- CT scan is recommended when complete removal of pleural fluid is not possible
- Thoracoscopy can be used to make a diagnosis of malignancy
- Routine flexible bronchoscopy is not recommended for pleural effusions
Prognosis
The prognosis depends on the cause of the pleural effusion. Benign effusions can be cured but if the cause is a malignancy, the prognosis is very poor. Another feature of pleural effusions is recurrence which can also occur with benign disorders like lupus, uremia, and rheumatoid arthritis. If the pleural effusion is not drained it can lead to dyspnea and even an empyema.
Consultations
- Pulmonologist
- Thoracic Surgeon
Pearls and Other Issues
If a large pleural effusion is drained quickly and volumes of more than 1.5 L are removed, the rapid re-expansion of the collapsed lung may occasionally lead to re-expansion pulmonary edema. Pleural manometry and monitoring pleural pressure during drainage of large volumes and terminating further drainage once the pleural pressure drops below -20 cm water or terminating with the onset of chest pain may prevent re-expansion pulmonary edema.
Enhancing Healthcare Team Outcomes
Because of the many causes of pleural effusions, it is important to have an interprofessional team. Irrespective of the cause, if the fluid is aspirated or a chest tube is inserted, the nurse has to look after the patient to ensure that he or she has not developed a pneumothorax. A chest x-ray is mandatory after pleural fluid aspiration. Once a chest tube is inserted, the nurse is responsible for recording the drainage, ensuring a proper seal of the connections and assessing the patient's respiratory status. Some patients with a chest tube or a pleural effusion may require oxygen and chest physical therapy. For those with chylous ascites, a dietary consult is necessary to help lower the amount of drainage. Limiting fat intake may benefit some patients. Regular chest x-rays are done in patients with chest tubes, and thus the radiologist has to evaluate the chest tube position and site of drainage ports. Pain is a significant problem when chest tubes are inserted, and hence, anesthesia may be contacted for a pain pump or a thoracic epidural.[14][15]
Clinicians need to inform patients with recurrent pleural effusions about the treatment options and their potential side effects. Open communication between the team members is necessary to improve patient outcomes and reduce morbidity.
Outcomes
The outcome of patients with pleural effusions depends on the cause, severity and patient comorbidity. In general, people who do not seek therapy have a poor outcome compared to those who are treated. Overall, patients with malignant pleural effusions tend to have a poor outcome. Most patients are dead within 12-24 months, irrespective of the cause of the malignant pleural effusion. When pleural effusions are inadequately treated, this can result in an empyema, sepsis and even a trapped lung. [9][10](Level V)
(Click Image to Enlarge)