Fascioliasis
- Article Author:
- Ryan Good
- Article Editor:
- Dmitriy Scherbak
- Updated:
- 5/21/2020 10:50:47 AM
- For CME on this topic:
- Fascioliasis CME
- PubMed Link:
- Fascioliasis
Introduction
Fascioliasis is a parasitic infection primarily of the hepatobiliary system caused by one of 2 digenean flatworms, Fasciola hepatica or Fasciola gigantica, which are commonly referred to as liver flukes.[1]
Etiology
Adult Fasciola hepatica flukes grow to become spiny-appearing, brown leaf-shaped, flatworms, typically around 30 mm by 15 mm in size and easily seen with the naked eye. Anteriorly, they have 2 suckers, a large one on the ventral side referred to as the acetabulum that allows the fluke to suction itself to the wall of the bile duct and remain in place so the smaller more anterior sucker may feed on the bile.[2] As their name implies, Fasciola gigantica flatworms can grow up to 75 mm in length but are otherwise similar in appearance to their smaller counterparts.[3][4]
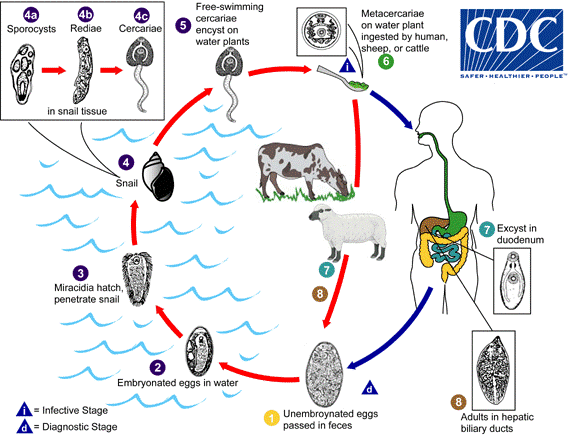
Regardless of whether the host is bovine or human, the life cycle remains the same. Adult liver flukes release un-embryonated eggs into their host’s bile ducts which then pass into their stools. The host defecates in the environment near or within a pond or steam. Eggs become embryonated when they reach a water source and hatch into free-swimming miracidia that seek out an intermediate host, one of several species of amphibious snail. The miracidium will bore itself directly into the tissues of the snail. Once inside, miracidia go through several metamorphoses first becoming sporocysts that give rise to mother rediae which in turn produce daughter rediae. Germ balls develop inside the daughter rediae that become cercariae which will grow and bore back out of the snail to again become free-swimming in the environment. They go on to encyst as metacercariae on aquatic vegetation and are eaten by their definitive host.[5]
The flatworms most commonly cause infection when humans when they eat vegetation contaminated with metacercariae, for example, watercress. Metacercariae excyst in the duodenum, and similarly in the snail, and penetrate through the intestinal wall into the peritoneum. From there, they migrate cephalad, boring through the parenchyma of the liver before finally settling in the biliary ducts. In 3 to 4 months they develop into adults, occupying the large biliary ducts primarily and laying more than 20,000 eggs per day. Adults can live up to 13 years in a human host if untreated.[2]
The migrating metacercariae cause parenchymal liver damage, setting off a cascade of inflammatory and immune responses leading to a constellation of acute symptoms. Adult flukes may partially or completely obstruct the bile ducts, over time causing fibrosis, hypertrophy, and later dilation of the proximal biliary tree. Parasite load is typically positively correlated with the degree of liver damage.[1]
Epidemiology
The global prevalence of Fasciola infection is estimated between 2.4 and 17 million and is typically under-reported and under-diagnosed. In the United States, sporadic cases are seen mainly in travelers and immigrants. Disease in cattle and livestock is more prevalent in the United States and varies in geographical location.[6][7] Fasciola hepatica is endemic to Europe and Asia, occasionally seen in Northern Africa, Central and South America, and the Middle East, and sporadic cases rarely pop in the United States and the Caribbean.[8] Fasciola gigantica infects domestic livestock across Asia, the Pacific Islands, and some parts of Northern Africa.[3] In parts of Africa and Asia, the 2 species overlap, and their clinical presentations are indistinguishable. Cattle and sheep are the most common definitive hosts, but the flatworms often infect several other grazing animals in the wild. Humans who live in regions where cattle and sheep industries are prominent and who consume raw aquatic vegetation, watercress in particular, are at the highest risk of contracting the parasite.[1]
History and Physical
There are 2 distinct phases of the fascioliasis infection.
The acute (hepatic) phase usually begins 6 to 12 weeks after ingestion of metacercariae from a contaminated water source. The first sign is usually very high fever, followed by right upper quadrant pain, hepatomegaly, and occasionally jaundice. Cell blood count (CBC) differential will show a marked peripheral eosinophilia. Patients often present with associated myalgias, urticarial rash, nausea, anorexia, and diarrhea. These symptoms are attributed to the Fasciola flatworms migrating through the liver parenchyma and setting off the inflammatory and immune responses along the way. Additional laboratory diagnostic clues include transaminitis, anemia, elevated erythrocyte sedimentation rate (ESR), and hypergammaglobulinemia. Early complications are rare but may be seen with high parasite load and include ascites, hemobilia, subcapsular hematomas, and rarely severe parenchymal liver necrosis. However, in most cases, acute symptoms generally resolve within 6 weeks, and the infection settles into the chronic phase.[1]
Early extrahepatic manifestations are typically type III (immune complex deposition) or type IV (IgE) hypersensitivity-mediated. A reactive eosinophilic pneumonitis is nonspecific for helminth infections and is well described in literature.[9] Overwhelming activation of the host’s immune system and inflammatory response can cause vasculitis. In the heart can lead to myocarditis which in turn can cause cardiac conduction abnormalities. In the brain cerebral vasculitis may lead to focal neurologic deficits or seizures but these are all highly uncommon. Generalized lymphadenopathy is often present.[1]
The chronic (biliary) phase usually begins about 6 months after acute infection once the flukes have settled into the bile ducts and may last up to a decade or more. Usually asymptomatic, but occasionally can present as chronic epigastric and right upper quadrant pain, nausea, vomiting, diarrhea, hepatomegaly, jaundice, and failure to thrive. Peripheral eosinophilia will not likely be present. Chronic common bile duct obstructions may develop and lead to recurrent jaundice, cholelithiasis, pancreatitis, and more seriously, ascending cholangitis. Prolonged infection and/or high parasite load leads to chronic hepatobiliary damage and can result in chronic biliary cirrhosis, sclerosing cholangitis, and even cholangiocarcinoma.[1][10][11]
Fasciola’s migration out of the intestine and up into the liver is not always perfect; sometimes they get lost. Ectopic fascioliasis is the term given to the infection occurring outside the hepatobiliary system. They may end up almost anywhere in the body. Their presence results in localized mononuclear, eosinophilic infiltration that causes secondary tissue damage from the host’s immune response. Frequently, when they get lost, they end up in the subcutaneous tissue of the abdominal wall, but they may also end up in the peritoneum, intestinal wall, lungs, heart, brain, muscles, or eyes. Traveling flatworms under the skin or fat tissue leave behind migrating, pruritic, erythematous, painful nodules 1 to 6 cm in diameter and may be seen or palpated. Occasionally nodules can become infected and turn into a local abscess.[1][12][13][14][15] Another rare form of ectopic fascioliasis is Halzoun syndrome or pharyngeal fascioliasis. It is sometimes seen in the Middle East in areas where people eat raw liver. In this case, the metacercariae or young adult flukes living in the ingested liver attach themselves to the mucosa of the upper respiratory or digestive tract causing pharyngitis or esophagitis. Rarely edema and swelling of the upper airway can be so severe that patients have asphyxiated.[16]
Evaluation
Fascioliasis is exceedingly rare in the United States, but should be on the differential for any patient with the combination of abdominal pain (especially right upper quadrant), transaminitis, and marked peripheral eosinophilia. Even more suspicion should arise if a patient has traveled to endemic areas of Europe, Asia, or the Pacific, and their dietary history includes watercress ingestion or consumption of raw vegetables washed in potentially contaminated water. Often there is a delay in the diagnosis of this disease even in endemics areas due to its rarity and nonspecific acute symptoms.[2][17]
Serology has become the fastest and most efficient way to test for fascioliasis in recent years. Titers against fasciola antigens become positive during the early phase of parasite migration and are detectable 2 to 4 weeks following initial exposure to the host’s immune system. Enzyme-linked immunosorbent assay (ELISA) techniques have largely replaced stool ova and parasite testing because of its fast turn-around, sensitivity, and quantification.[18][19] Serum fasciola antigen levels often positively correlate with the degree of infection.[20] It also takes 5 to 7 weeks after initial infection before adult worms are mature enough to produce eggs; thus, there are no eggs in stool during the acute phase of the infection. Antigen levels positively correlate with the infectious burden. Successful treatment and extermination of the parasites correlate with a decline in the ELISA titers to fasciola antigens. Antibodies may still be detectable at low levels for years. The assay becomes undetectable in approximately 65% of patients one month following successful treatment. Some patients will have a low positive titer for life without any evidence of active infection.[21][22]
Microscopic examination of stool or duodenal bile aspirates for eggs is still useful for detecting chronic infections in rural or third world settings where resources can be scarce. They are also sometimes found incidentally while working up biliary obstructions. Fasciola hepatica eggs are yellow-brown and ovoid measuring 130 to 150 micrometers long by 60 to 90 micrometers wide. A single adult fluke can release over 20,000 eggs per day, but that release is intermittent. Thus, an examination of multiple stool or duodenal aspirate specimens may be needed as one negative examination does not necessarily exclude the diagnosis.[23]
Rarely, one might also identify adult flukes in the biliary tree during endoscopic retrograde cholangio-pancreatograhy (ERCP) during assessment in a patient with biliary obstruction. Occasionally capsular liver nodules can be seen by a surgeon during a diagnostic laparoscopy, but with no further clinical history are nonspecific. Liver biopsy is not routinely warranted as serology if much more specific, sensitive, and cost effective.[24]
Computerized Tomography scan may show multiple, nodular, small (approximately 25 mm in diameter), branching, subcapsular lesions in the parenchyma of the liver. These tortuous tracks are left behind by the migrating parasites. Necrotic areas may be seen on scans utilizing inravaenous (IV) contrast.[24][25][26] Subcapsular hematoma, capsular thickening, or parenchymal calcifications can also be seen.[27]
Ultrasound, cholangiogram, and ERCP are helpful during the biliary stage and may show the mobile, leaf-like flukes in biliary ducts or gallbladder, and are often associated with stones. Irregular common bile duct wall thickening, hepatomegaly, splenomegaly, and/or peri-portal lymphadenopathy are often present, especially in acute phase.[24]
Treatment / Management
The treatment of choice is oral triclabendazole 10 mg/kg daily taken after a meal for 2 days. Triclabendazole is an imidazole derivative and works by preventing the polymerization of tubulin into microtubules rendering cells incapable of producing their cytoskeletal structures. It is effective against all stages of fascioliasis with a cure rate of over 90%.[28][29] Fasciola species do not respond well to ivermectin, praziquantel, artesunate, mebendazole, or albendazole.[30]
Differential Diagnosis
Acute hepatitis, autoimmune hepatitis, and shock liver will all have very significantly elevated transaminases, typically in the thousands. This is much high than the levels seen in fascioliasis which are usually in the hundreds.
Toxocariasis, acute schistosomiasis, ascariasis, and strongyloidiasis, are all nearly indistinguishable from fascioliasis in the acute phase, especially in the presence of peripheral eosinophilia and pulmonary symptoms. Serologic testing is required to rule these out.
Prognosis
Complications
Chronic infections can be complicated by ascending cholangitis which requires IV antibiotics and usually surgery. Biliary obstruction by parasites in the setting of ascending cholangitis requires emergency endoscopic retrograde cholangiopancreatography (ERCP) for the direct removal of the worms from the common bile duct if possible. Chronic infections lead to chronic biliary inflammation and destruction giving rise to sclerosing cholangitis, biliary cirrhosis, and cholangiocarcinoma.[10][11][5]
Deterrence and Patient Education
Follow-up after treatment should assess for a decrease in serologic titers, resolution of eosinophilia, and clearance of eggs in the stool. It is reasonable to repeat all positive tests at 3 months post-treatment. Resolution of biliary findings on ultrasound after therapy may also be helpful if the patient presented with biliary obstruction as scar tissue and biliary duct thickening may still be present and can cause prolonged symptoms.[31]
When a patient is diagnosed with fascioliasis, other family or house members should be tested for serum fasciola antigens. Any patient who tests positive, regardless if they are symptomatic or not, should be treated to avoid the risk of future complications.
Infection can be prevented by educating patients to avoid ingestion of raw freshwater plants, especially in endemic areas. Elimination of snail intermediates have been attempted in endemic areas in the past but is not practical. There are no vaccines available for humans and the disease is much too rare in the human population for one to be economically beneficial.
Enhancing Healthcare Team Outcomes
Management of fascioliasis starts with early recognition of this disease that often goes undiagnosed. It requires a high degree of suspicion and understanding that serologic evaluation is the most beneficial and cost-effective analysis to assess for the disease.[17]
Coordination of care requires a team of healthcare providers that includes physicians, often primary care hospitalists along with gastroenterologists and often infectious disease specialists. Nurses, laboratory technicians, and pharmacists also play a part in the handling and evaluation of specimens and the delivery of the care to the patient.[28]