
Neuroanatomy, Globus Pallidus
- Article Author:
- Nismat Javed
- Article Editor:
- Marco Cascella
- Updated:
- 7/31/2020 3:18:12 PM
- For CME on this topic:
- Neuroanatomy, Globus Pallidus CME
- PubMed Link:
- Neuroanatomy, Globus Pallidus
Introduction
The globus pallidus (GP) is one of the components of basal ganglia. It divides into globus pallidus internus (GPi) and globus pallidus externus (GPe). The globus pallidus and putamen collectively form the lentiform (lenticular) nucleus, which lies beneath the insula. The globus pallidus, caudate, and putamen form corpus striatum. The corpus striatum is also an important part of basal ganglia. The thalamus, subthalamus, and substantia nigra (SN) are not a part of basal ganglia but serve essential functions for the network.
The motor system controlled by basal ganglia is made of corticobulbar and subcortical structures, the gray matter of the spinal cord, cerebellum, and efferent nerves. The basal ganglia coordinate with other structures in the brain to plan and implement goal-oriented behaviors. This co-ordination requires multiple striatal (motor), cognitive, and limbic (reward) circuits and pathways. The globus pallidus can modulate these pathways because of its connections. The major output of striatum is through the GPe. The GPi acts as the final output for both direct and indirect pathways of the basal ganglia network. The thalamus, however, is slightly different. It acts as a relay because of its reciprocal interconnections with cortical and subcortical structures. Therefore, the thalamus can perform multiple motor and sensory functions. These unique characteristics enable each component to work effectively.
The dysfunction of the GP has been noted in ischemia, alcohol, and opiate abuse. This dysfunction gives rise to various cognitive and motor problems.
Structure and Function
The GP is a subcortical structure of the brain. It is a triangular mass of cells located medial to the putamen. The pale appearance of the GP is due to the myelinated axons that make up the structure; this is in contrast with the unmyelinated structures (putamen and striatum), which appear darker. The medial medullary lamina of the white matter divides the GP to globus pallidus internus (GPi) and globus pallidus externus (GPe).[1] The GPi is the medial subdivision, whereas GPe is the lateral subdivision. The GPe is relatively larger than GPi. It is also located caudomedially to the corpus striatum.[2]
The main function of the globus pallidus is to control conscious and proprioceptive movements. The GPe is the intrinsic nucleus, whereas the GPi is the output nucleus. The intrinsic nucleus acts as a relay for information. The output nucleus, primarily, sends information to the thalamus.[3] To perform its function effectively, the GP receives input from multiple structures. The GP receives the majority of the inhibitory input (GABAergic) from the striatum. It is important to note that substantia nigra pars compacta (SNPc) has projections to the striatum. These projections secrete dopamine to target either D1 or D2 receptors present on the striatum. D1 receptors are excitatory (glutamatergic) in nature, whereas D2 receptors are inhibitory in nature.[4] Some inhibitory input (GABAergic) also comes from the caudate and putamen. The output is computed through the direct and indirect pathways.
The ‘direct pathway’ promotes movement. The SNPc excites the striatum by secreting dopamine (nigrostriatal projections) to activate the D1 striatal receptors. The excited D1 receptors activate the striatum, and more inhibitory signals are sent to the GPi. A few inhibitory signals transmit simultaneously to the substantia nigra pars reticularis (SNPr). As a result, the GPi and SNPr experience inhibition. The GPi and SNPr lose their inhibitory influence on the thalamic nuclei (ventral anterior thalamic nucleus and ventral lateral thalamic nucleus). This loss of influence means that the thalamic nuclei are no longer inhibited and can send excitatory signals to the motor cortex via corticospinal projections. Ultimately, the excitation of the motor cortex allows the required movement to occur. It is important to note that GPi secretes an opiate known as substance-P, which may regulate the nigrostriatal pathway.[5]
The ‘indirect pathway’ limits motor function so that excessive and erratic movements do not occur. The SNPc inhibits the striatum by secreting dopamine (nigrostriatal projections) to activate D2 striatal receptors, which means that the inhibited D2 receptors send less inhibitory signals to GPe. The GPe is in a relatively excited state. Therefore, it sends more inhibitory signals to the subthalamic nucleus. The inhibited subthalamic nucleus can no longer inhibit the GPi and the SNPr. The GPi and the SNPr are, therefore, able to inhibit the thalamic nuclei. Ultimately, the thalamic nuclei inhibit movement. In recent experiments, the GPe secrete an opiate called enkephalin.[6] This opiate can modulate the nigrostriatal pathway.[7]
These pathways, therefore, explain how the GPe and the GPi differ in their functions.
Embryology
Human brain development begins during the third week of gestation from the ectoderm. The notochord induces neurulation, a process by which ectoderm above the notochord forms neuroectoderm to give rise to other structures. As a result, the neural tube begins to form. The brain continues to develop from the neural tube. Following the closure of this neural tube by week 6, the primary brain vesicles form. These primary brain vesicles include the prosencephalon (forebrain), mesencephalon (midbrain), and rhombencephalon (hindbrain).[8] These primitive brain vesicles continue to differentiate into different structures. In the fifth week of gestation, the prosencephalon develops into the telencephalon and the diencephalon. The telencephalon has dorsal and ventral parts. The dorsal telencephalon gives rise to the cerebral cortex, whereas the ventral telencephalon gives rise to the basal ganglia.[9] Thus, it is from the ventral telencephalon that the GP develops.
Blood Supply and Lymphatics
The GP receives its blood supply from the anterior choroidal artery (AChA), middle cerebral artery (MCA), and anterior cerebral artery (ACA). The MCA provides the majority of the blood supply. The perforating branches of the ACA supply the anterior and inferior portion of the GP. The perforating arteries from the MCA perfuse the majority of the superior and posterior portion of the lateral section of the GP. Heubner’s artery and ACA also supply [10][11] A small portion of the lateral segment of the GP. The AChA perfuses the medial portion of the GP.[10]
The venous drainage of the GP derives from veins that drain the adjacent basal ganglia, particularly caudate and putamen. The system forms from a deep and ventricular group of veins. The deep group subdivides into a group of inferior striate veins that drain the putamen and caudate nuclei. The deep group of veins joins the deep middle cerebral and basal veins. The ventricular group of veins drains the caudate nucleus. The ventricular group joins the internal cerebral and basal veins.[11] The basal and internal cerebral veins join to form the great vein of Galen, which then drains into the straight sinus.
The GP, as a part of basal ganglia, has a similar lymphatic drainage system as the cerebral cortex. The lymphatic system of the cerebral cortex forms from perivascular, cerebrospinal fluid (CSF), and olfactory drainage pathways as well as meningeal lymphatic vessels.[12][13]However, a study mentions that the perivascular spaces in the basal ganglia and cerebral cortex are structurally different. This difference is partly responsible for the difference in fluid composition between the two.[14]
Nerves
The globus pallidus, being a part of basal ganglia, plays an additional role in higher cognitive functions such as reinforcement and memory building. The nuclei (including GP) and cortical areas interconnect via independent parallel loop circuits. The association and limbic cortices project to specific striatal domains, which, in turn, project back to the corresponding cortical areas via the globus pallidus and the thalamus. There have also been suggestions that the location of both the GP and substantia nigra helps in the transmission of information from the limbic to the associative system.[15]
Physiologic Variants
Surgical Considerations
The GPi has implications in the surgical procedures that involve deep brain stimulation (DBS) for the treatment of dystonia in disorders like Wilson’s disease to improve quality of life.[19][20] A study noted that the DBS of bilateral GPi could be an effective treatment for dystonia due to the GNAL mutation, especially in patients unresponsive to medication or botulinum toxin.[21] With depression being among the most common non-motor symptoms of PD, some investigators have reviewed the evidence to determine if there is a difference in mood effects (depression) after the DBS of either GPi or STN. However, their review found no clear benefit of targeting either the STN or the GPi.[22]
Since GPi is the principal striatal output circuits, it has seen significant use in ablative surgery for a variety of neuropsychiatric disorders. The GPi surgery has long been the target for the treatment of hyperkinetic and hypokinetic movement disorders, including behavioral disorders such as Tourette’s syndrome. Pallidotomy was a surgical intervention for Huntington disease and Parkinson disease (PD). However, pallidotomy was abandoned in the mid-20th century as treatment of PD.
Clinical Significance
Studies have demonstrated that the involvement of GPi in different disorders, such as obsessive-compulsive disorders (OCD), Tourette’s syndrome, acquired dystonia, and attention deficit hyperactivity disorder (ADHD). Alterations in the resting-state functional connectivity of the cortico-striatal circuits have been implicated in OCD. Indeed, recent studies have noted increased resting-state functional connectivity between the left GPe and the left STN. This increased connectivity was also present between the left GPe and the left GPi. The involvement of these structures in the pathophysiology provides evidence for success in DBS targeted at GPi.[23]
The GP also has implications in the parkinsonian motor symptoms. Studies have elucidated that dopamine depletion leads to excessive stimulation (increase in the firing rate) of the GPi neurons causing parkinsonian motor symptoms, as evidenced by increased activity of GPi in PD.[24] Other studies of the PD model have also shown that the loss of striatal dopaminergic activity leads to the disinhibition of the D2-striatal projection neurons, which causes increased inhibition of the GPe.[24] The inhibition of GPe, in turn, causes the disinhibition of the subthalamic nucleus, which results in the downregulation of autonomous firing in the STN neurons. Additionally, neuroimaging studies of the patients with Huntington’s disease have reported a severe decrease in the size of the GP.[25]
Unlike the putamen and caudate, the GP is spared from ischemic injury after a hypoxic-ischemic insult. There are also case reports of bilateral GP lesions following alcohol and opiate abuses.[26] Since the GP is a critical structure in the basal ganglia circuits, its understanding is essential to gain a better understanding of motor and non-motor dysfunctions of the circuits.
(Click Image to Enlarge)
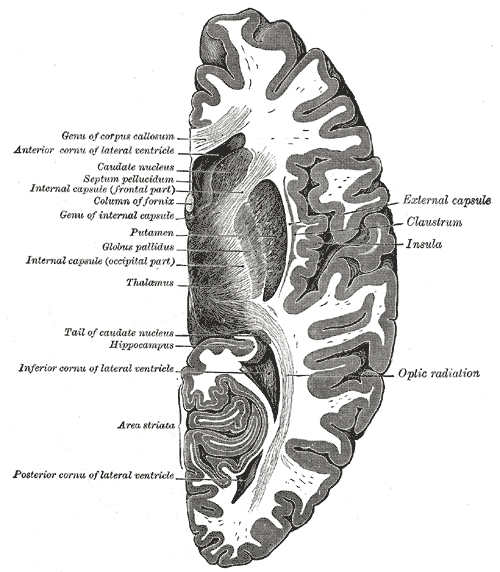
Horizontal section of right cerebral hemisphere, Genu of Corpus callosum, Anterior cornua of lateral ventricle, Caudate nucleus, Septum pellucidum, Internal capsule (frontal part), Column of fornix, Genu of internal capsule, Putamen, Globus pallidus, Internal capsule (occipital part), Thalamus, Tail of caudate nucleus, Hippocampus, Inferior cornua of lateral ventricle, Area striata, Posterior cornua of lateral ventricle, Optic Radiation, Insula, Claustrum, External capsule
Contributed by Gray's Anatomy Plates
(Click Image to Enlarge)

Coronal section of brain through anterior commissure, Caudate nucleus, Internal Capsule, Putamen, Globus pallidus, Claustrum, Insula, Optic Chiasma, Third Ventricle, Anterior Commissure, Columns of fornix, Cavity of septum pellucidum, ANterior Cornu, Corpus callosum
Contributed by Gray's Anatomy Plates
(Click Image to Enlarge)
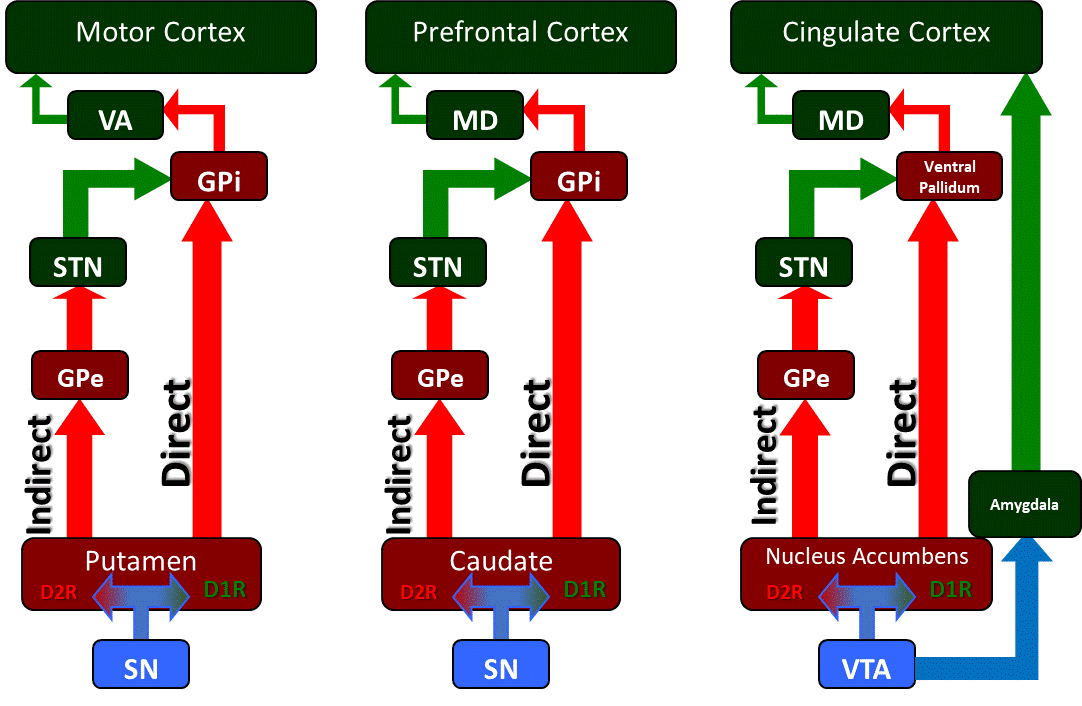
The basal ganglia circuitry and its associated motor, cognitive, and limbic outputs. The basal ganglia is a collection of subcortical nuclei that regulate various cortical functions including voluntary motor control, cognition and executive functions including planning, and limbic functions related to fear and reward processing. The substantia nigra (SN) and ventral tegmental areas (VTA) are dopaminergic inputs into regions of the striatum which include the putamen, caudate, and nucleus accumbens. These striatal populations contain two populations of dopamine-receptive families, D1-family receptor expressing neurons and D2-family receptor expressing neurons. The D1-family neuron populations are part of the "Direct" pathway that send projections directly to the globus pallidus internus (GPi), while the D2-family neurons are part of the "Indirect" pathway that sends signals through the globus pallidus externus (GPe) to the subthalamic nucleus (STN) to the GPi.
Contributed by James W. H. Sonne, PhD
(Click Image to Enlarge)

The basal ganglia circuitry as it is affected in Parkinson's disease. Neurodegenerative loss of the substantia nigra (SN) and its dopaminergic projections to the putamen lead to a decrease in activity in the direct pathway and an increase in activity in the indirect pathway. As a result, the subthalamic nucleus (STN) disproportionately stimulates the globus pallidus internus (GPi) which in turn inhibits the ventral anterior (VA) nucleus of the thalamus and a loss of activitation of the motor cortex and its corticospinal projections to the spinal cord alpha motor neurons. These patients experience deficits in the initiation of motor movement along with poor balance control and changes in gait, along with other signs and symptoms.
Contributed by James W. H. Sonne, PhD