Gout
- Article Author:
- Ardy Fenando
- Article Editor:
- Jason Widrich
- Updated:
- 8/8/2020 8:15:58 PM
- For CME on this topic:
- Gout CME
- PubMed Link:
- Gout
Introduction
Gout is among the most prevalent etiologies of chronic inflammatory arthritis in the United States, which is characterized by monosodium urate (MSU) monohydrate crystals deposition in the tissues.[1][2] Gout was first recognized even before the common era. Hence it is arguably the most understood and manageable disease among all rheumatic diseases.[3][4]
Etiology
Risk Factors
Hyperuricemia is the leading cause of gout.[1][5] People with higher serum urate levels are not only at an elevated risk for gout flare-ups but will also have more frequent flare-ups over time. In a study of more than 2000 older adults with gout, those with levels more than 9 mg/dl were three times more likely to have a flare over the next 12 months than those with levels less than 6 mg/dl.[6]
Hyperuricemia is not the only risk factor for gout. In fact, only a minority of these patients go on to develop gout. Other factors implicated for gout and/or hyperuricemia include older age, male sex, obesity, a purine diet, alcohol, medications, comorbid diseases, and genetics. Offending medications include diuretics, low dose aspirin, ethambutol, pyrazinamide, and cyclosporine. Genome-wide association studies (GWAS) have found several genes that are associated with gout. These include SLC2A9, ABCG2, SLC22A12, GCKR, and PDZK1. [1],[7] Dietary sources that can contribute to hyperuricemia and gout include consumption of animal food such as seafood (e,g., shrimp, lobster), organs (e.g., liver, and kidney), and red meat (pork, beef). Some drinks like alcohol, sweetened beverages, sodas, and high-fructose corn syrup may also contribute to this disease.[1]
Triggers
Every condition that causes alterations in extracellular urate concentration has the potential to trigger a flare-up. These conditions include stress (surgical procedure, recent trauma or starvation), dietary factors (e.g., fatty food, beer, wine, and spirits), and drugs (e.g., aspirin, diuretics, or even allopurinol).
Epidemiology
The prevalence of gout can vary by age, sex, and country of origin. In general, the prevalence of gout is 1 to 4%. Older age and male sex are two common risk factors noted globally. In western nations, the prevalence of gout in men (3 to 6%) is 2 to 6 fold higher than in women (1 to 2%). Prevalence increases with age but plateaus after 70 years of age. In 2007 to 2008, around 3.9% of US adults received a diagnosis with gout.[8] The prevalence of gout is also higher among individuals with chronic diseases such as hypertension, chronic kidney disease, diabetes, obesity, congestive heart failure, and myocardial infarction.
Comorbidities[9]:
Hypertension, diabetes, hyperlipidemia, and metabolic syndrome are often associated with gout. Individuals with psoriasis have increased urate production and are prone to gout. On the other hand, patients with renal insufficiency have decreased urate excretion, which also results in gouty attacks.
Pathophysiology
Hyperuricemia
Hyperuricemia is the key factor for the development of gout as it can promote monosodium urate crystal nucleation and growth by reducing urate solubility. Uric acid in the blood comes from both exogenous and endogenous purine breakdown, which then gets excreted through the kidneys. Overproduction and/or underexcretion of uric acid is the foundation for the rise of serum uric acid levels.[5]
Inflammatory Response
Inflammation starts when macrophages phagocytize monosodium urate crystals and trigger the formation and activation of cytosolic protein complexes (NLRP2 inflammasome).[10] These complexes subsequently recruit caspase-1 which activates pro-IL-1beta to IL-1beta. IL-1beta plays an important role in the inflammatory response to gout.[3][10][11] It promotes vasodilatation, recruitment of monocytes and initiates, as well as, amplifies the inflammatory cascade. Further IL-1beta secretion can result in bone and cartilage break down. Other cytokines, such as TNF-1, IL-6, CXCL8, and COX-2, are also involved in the inflammatory response.[11]
Histopathology
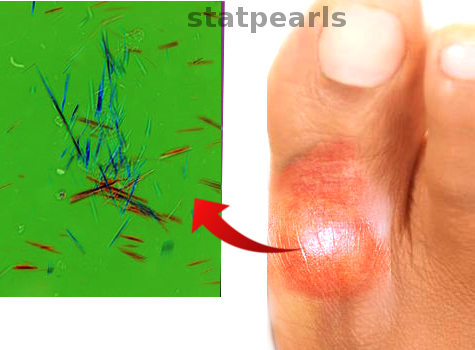
Monosodium urate crystal deposition, under polarizing light microscopy, is typically described as a rod or long needle-shaped crystals with negative birefringence.[12] Under light microscopy, tophi will consist of several zones; the crystalline center, the surrounding corona zone, and then the fibrovascular zone. Multinucleated giant cells, histiocytes, and plasma cells are present in the corona zone.[13]
History and Physical
In a patient who is having a gout flare-up, the symptoms are often apparent. The most commonly involved joint is the first metatarsophalangeal joint. The talar, subtalar, ankle, and knee can also be involved in some cases. Although affliction of the joints mentioned above is common in gout, the physician should pay attention to other joints, specifically those joints with underlying osteoarthritis. Besides joints, other periarticular structures such as tendons and bursa may also be affected.[1]
Patients usually present with acute onset of joint pain. The pain is often sudden waking the patient from sleep or may have developed gradually over a few hours before the presentation, with the maximum intensity of pain reaching at 24 hours.[14] The pain is usually severe and not responsive to the usual home remedies; even touching the joint can be excruciatingly painful. Gout flare-ups often incite local inflammation which presents as erythematous, swollen and a warm joint. Systemic features of the joint inflammation may include fever, general malaise, and fatigue.[1]
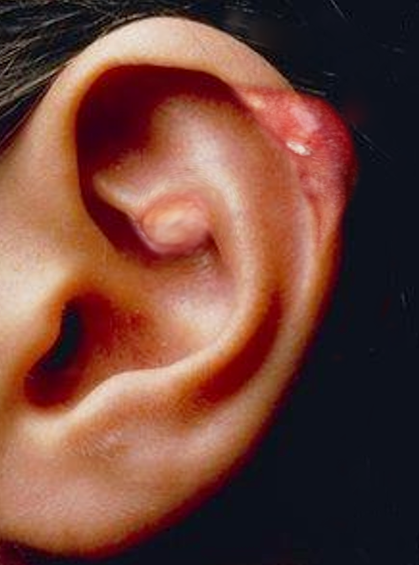
The physical exam findings align with the patient history. The affected joint is typically red, swollen, warm, and tender.[15] In patients with chronic gout, the flare-up may involve multiple joints. With the involvement of many joints, it can cause a systemic inflammatory response syndrome that may masquerade as sepsis.[16] Tophi, which are subcutaneous depositions of urate that form nodules, can also be found in patients with persistent hyperuricemia. Tophi typically occur in the joints, ears, finger pads, tendons, and bursae.[1]
Evaluation
Synovial Fluid Analysis
Monosodium urate crystal identification remains the gold standard for gout diagnosis. Synovial fluid during a gout flare-up usually is yellow in color and cloudier in appearance. It contains crystals and white blood cells. In patients with septic arthritis, the synovial fluid will be more opaque with yellow-green appearance. Under a microscopic examination, synovial fluid for septic arthritis will have a higher white blood cell count (over 50000/ml) than in gout, and a positive gram stain. Additionally, cultures will be positive for bacteria and negative for crystals.
Synovial fluid or tophus aspiration analysis under polarizing microscopy reveals needle-shaped, negatively birefringent crystals.[1][3][17] Arthrocentesis is also necessary to confirm the diagnosis and rule out other septic arthritis, Lyme disease, or pseudogout (calcium pyrophosphate).[17]
Laboratory Study
The examination usually reveals elevations in the white blood cell count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP) during a gout flare-up but these features are non-specific and do not confirm the diagnosis.
During an acute gout flare-up, serum urate may also be in the normal range. The physician should repeat the serum urate level in patients with an uncertain gout diagnosis after the resolution of the flare-up. Hyperuricemia is helpful in the clinical diagnosis of gout in symptomatic patients, but hyperuricemia alone does definitively confirm the diagnosis. Asymptomatic hyperuricemia is not uncommon in the general population. Persistently low serum urate concentrations make the diagnosis of gout less likely.[3]
Urinary fractional excretion of uric acid can be measured, especially in young populations with a non-specific cause of hyperuricemia. It will help to differentiate between overproduction or under excretion of uric acid and can act as a guide for therapy.
Imaging
Although not routinely used, ultrasonography and dual-energy CT (DECT) can assist in the diagnosis of gout. Monosodium urate deposition will be apparent on ultrasound as a hyperechoic enhancement over the cartilage; also known as a double contour sign. DECT can identify urate due to the beam attenuation after exposure to two different X-ray spectra.[1][3]
Treatment / Management
The treatment of gout is based on the goals of treatment. During acute flares, the goal is solely to reduce the inflammation and symptoms. Long term goal is to reduce serum urate levels to achieve suppression of flare-ups and regression of tophi.[3]
Acute Flares
Management of acute flares aims at decreasing the inflammation and the resulting pain. The physician should start the treatment within the first 24 hours of onset to reduce the severity and duration of the flare-up.[5]
Non-pharmacological management such as rest with topical application of ice packs can combine with medications that reduce inflammation. First-line treatment for gout flares are NSAIDs, colchicine, or systemic glucocorticoids. The length of the treatments should be at least 7 to 10 days to prevent rebound flare-up.[18][19][20]
There is no data to favoring one NSAID over the other. High dose, fast-acting NSAIDs such as naproxen or diclofenac are options. Indomethacin is not preferable due to its toxicity profile.[5]
Colchicine has been shown to reduce the pain by over 50% in a randomized control trial at 24 hours when compared to a placebo. The FDA recommends an initial dose of 1.2 mg, followed by 0.6 mg one hour later. A prophylactic dose (0.6 mg once or twice daily) should be given twelve hours after the last dose. [1] Older populations, patients with chronic kidney disease (CKD) and/or hepatic impairment, require dose reduction. Patients prescribed colchicine who are taking other drugs that affect cytochrome P450 (CYP) 3A4 and P-glycoprotein may need to stop or modify their medication regimen due to an increased risk of colchicine toxicity. The adverse effects of colchicine include gastrointestinal symptoms (nausea and diarrhea), myotoxicity, and myelosuppression (leukopenia, thrombocytopenia, and aplastic anemia).[21]
Glucocorticoids are a recommendation in patients who have gout but have contraindications to NSAIDs and/or colchicine. These agents are also drugs of choice for patients with renal insufficiency. Glucocorticoids can be administered intra-articularly for a monoarticular gout flare-up or orally for polyarticular flare-ups. IL-1 antagonists, such as anakinra, canakinumab, and rilonacept, are effective for acute gout flares but do not have approval for this use in the United States.[1]
Non-acute Flares
Pharmacologic
The clinician should not start urate-lowering therapy (ULT) in patients with asymptomatic hyperuricemia or gout with rare attacks (1 flare/year). The American College of Rheumatology (ACR) 2012, Guidelines for starting ULT include the following:
- Frequent flares (greater than or equal to two per year)
- CKD stage 2 or more
- Tophus diagnosis on physical examination or imaging
- Past urolithiasis.
Urate-lowering therapy is started at a low dose to monitor the side effects and response to treatment. Titration of the dose is every 2 to 6 weeks to achieve serum urate levels of less than 6 mg/dl or 5 mg/dl in those patients with tophi.[18]
During the initiation of ULT, there is an increased risk of gout flare-ups, so colchicine prophylaxis is recommended for 3 for months after achieving serum urate goal in the patients without tophi or 6 months with tophi to reduce the flare-up risk.[22]
ULT can categorize into three classes (based on the mechanisms).
- Xanthine oxidase inhibitors (XOI) - XOI works by inhibiting the synthesis of uric acid. This class includes allopurinol and febuxostat. Allopurinol is the recommended first-line pharmacologic ULT in gout.[18] The physician should monitor liver enzymes, renal, and blood count regularly. Adverse effects from allopurinol can range from skin rashes to life-threatening severe allopurinol hypersensitivity (especially in HLA-B*5801 positive patients).[1]
- Uricosuric - The uricosuric agents works by increasing renal urate clearance.[1] These agents are ineffective as monotherapy in patients with low creatinine clearance (less than 30 ml/minute) and contraindicated with patients with a history of nephrolithiasis. Drugs in this class include probenecid and lesinurad. Probenecid is the only agent approved for use as a monotherapy. Uricase is reserved only for refractory gout patients. Uricase works by converting uric acid into soluble allantoin. Pegloticase and Rasburicase are the approved uricase agents for treating hyperuricemia.[23] Patients have to stop taking other ULT agents while starting this therapy because they may develop antibodies against uricase which can only be detected by monitoring the serum urate level without being influenced by other therapy. This drug class also is contraindicated in people with glucose-6-phosphate dehydrogenase (G6PD) deficiency; hence, potential treatment warrants screening for this enzyme before initiating therapy.
- Interleukin-1 (IL-1) inhibitor - Interleukin (IL)-1 plays an important role in the inflammation in gout. Agents that block this interleukin, such as anakinra and canakinumab have an essential role, especially during gout flare-ups. There are still ongoing studies for this class. Therefore, these agents have do not yet have approval for gout flare-ups in the United States.
Non-pharmacologic
Patients with gout are encouraged to modify their lifestyles to prevent future attacks.
Diet recommendations include reducing alcohol consumption, limiting purine-rich foods (meat, seafood, high fructose corn syrup, and sweetened soft drinks) and substituting low-fat or non-fat dairy products for their higher fat content counterparts. Weight loss and adequate hydration will also help reduce gout flare-up frequency.
Differential Diagnosis
- CPPD deposition
- Septic arthritis
- Osteoarthritis
- Rheumatoid arthritis
- Psoriatic arthritis
- Cellulitis
Prognosis
The prognosis of gout depends on the comorbidity of each individual. Mortality is higher in individuals with cardiovascular comorbidity. When gout receives proper treatment, most patients will live a normal life with mild sequelae. For patients whose symptoms appear at an earlier age, they usually will have a more severe disease at presentation. For those who do not modify their lifestyle, recurrent flare-ups are common.
Complications
Tophi, Joint deformity, osteoarthritis, bone loss
Urate nephropathy and nephrolithiasis.
Gout might also cause ocular complications, such as conjunctivitis, uveitis, or scleritis from the urate crystal precipitation.[24]
Deterrence and Patient Education
Lifestyle changes are encouraged in gout patients, including weight loss, limiting alcohol intake, and avoiding certain foods. These changes will complement medical therapy but often are not enough alone to combat or reverse gout.
Enhancing Healthcare Team Outcomes
Most patients with gout have other comorbidities. The prevalence of gout is higher among individuals with chronic diseases such as hypertension, chronic kidney disease, diabetes, obese, congestive heart failure, and myocardial infarction.[9]
Gout treatment requires the collaboration of an entire interprofessional healthcare team approach. The physician (MD, DO, NP, PA) must promptly identify the pathology, and rule out differentials. Some cases may require a rheumatology consult. The pharmacological approaches to gout require consideration of these comorbidities and monitoring their response to treatment. The pharmacist and nurse both must educate the patient on medication compliance. Also, the pharmacist should assist the team by performing medication reconciliation, verify appropriate dosing, and consult on agent selection in the event of initial treatment failure. The dietitian should urge the patient to abstain from alcohol, avoid meat containing foods, and maintain healthy body weight. The role of specialists, primary care physicians, nurses, nurse practitioners, and dieticians are all critical in reducing gout morbidities. The medical team should coordinate education of the patient on lifestyle modification which can make a difference in reducing the risk and frequency of gout flare-ups.
All healthcare providers, including the primary care and nurse practitioners, should be able to identify classic gout symptoms and have a low threshold for referring the patients for an arthrocentesis if they are uncertain of the diagnosis. Then, working with the interprofessional team as outlined above, direct the treatment as needed and interact with the interprofessional team to drive outcomes. [Level 5]
Referral to a specialist/rheumatologist should be a consideration in the following patients with joint pain[18]:
- Unclear etiology with hyperuricemia,
- Unclear etiology with normal serum urate level
- Patients with renal impairment
- Failed trial of xanthine oxidase inhibitor treatment
- Multiple side effects from the medications
- Refractory gout [Level 1]
Only through an interprofessional team approach with close communication can the morbidity of gout be lowered.
![Gout, [SATA]](../pictures/6491.png)