Hand Nerve Compression Syndromes
- Article Author:
- Samir Sharrak
- Article Editor:
- Joe M Das
- Updated:
- 10/13/2020 11:15:11 AM
- For CME on this topic:
- Hand Nerve Compression Syndromes CME
- PubMed Link:
- Hand Nerve Compression Syndromes
Introduction
Nerve compression syndromes of the hand present with various signs and symptoms that correspond to the nerve involved and its anatomic distribution. There are three nerves and their corresponding branches that provide sensory and motor innervation to the hand that include the median, ulnar, and radial. An understanding of the anatomy and distribution of these nerves is paramount in distinguishing the various signs, and symptoms in nerve compression syndromes.[1][2]
The median nerve is a mixed motor and sensory nerve that forms from the convergence of the lateral and medial cords of the brachial plexus. It contains contributions from the anterior rami of C5-T1. It descends the anterior compartment of the arm alongside the brachial artery on the medial aspect. From there, it enters the forearm between the superficial and deep heads of the pronator teres muscle. At this point, it provides motor innervation to several muscles in the flexor compartment that include the pronator teres, flexor carpi radialis, palmaris longus, and the flexor digitorum superficialis. It continues to travel distally along the forearm between the flexor digitorum superficialis (FDS) and flexor digitorum profundus (FDP) muscles. As it continues distally, it gives off a branch called the anterior interosseous nerve, which supplies the deep forearm muscles that include: lateral half of the FDP that supply the second and third digits, flexor pollicis longus (FPL), and pronator quadratus. The median nerve then enters the hand via the carpal tunnel, along with the tendons of the FDS, FDP, and FPL. In the hand, it provides motor innervation to the flexor pollicis brevis (FPB), abductor pollicis brevis (APB), opponens pollicis, and the lateral two lumbricals. The sensory distribution of the median nerve supplies the palmar aspect of the lateral palm, palmar aspect of the lateral three and a half digits, and the dorsal aspect of the lateral three and a half digits distal to the PIP joint.[3]
The ulnar nerve is a mixed motor and sensory nerve that forms from the ventral rami of C8 and T1. It descends the arm medial to the brachial artery. It then passes posterior to the medial epicondyle of the humerus, into a passageway known as the cubital tunnel. It enters the forearm between the humeral and ulnar heads of the flexor carpi ulnaris (FCU) and continues down the forearm between the FCU and FDP. As it courses through the forearm, it gives off motor branches to the following muscles: FCU, medial FDP that supply the fourth and fifth digits. It enters the wrist lateral to the tendon of the FCU and enters a tunnel known as Guyon’s canal. In this canal, it bifurcates into a sensory branch and deep motor branch. The sensory branch provides sensation to the palmar aspect of the medial hand, fifth digit, and medial aspect of the fourth digit. The motor branch innervates the hypothenar muscles (abductor digiti minimi, opponens digiti minimi, flexor digiti minimi, and the palmaris brevis), the adductor pollicis, the deep head of the flexor pollicis brevis, the two medial lumbricals, and the dorsal and palmar interossei.[4]
The radial nerve is a mixed motor and sensory nerve that originates from the ventral rami of C5-T1. After emerging from the axilla, it travels posteriorly along with the profunda brachii artery in the posterior compartment of the arm. It traverses through the spiral groove between the lateral and medial aspects of the triceps muscle and further descends to the front of the lateral condyle of the elbow. During this course, It gives off multiple sensory nerves (posterior cutaneous nerve of the arm, inferior lateral cutaneous nerve of the arm, and the posterior cutaneous nerve of the forearm) that supply the posterior aspect of the arm and forearm. It also provides motor innervation to the following muscles of the arm: triceps muscle, anconeus, lateral brachialis, brachioradialis, extensor carpi radialis longus (ECRL), and the extensor carpi radialis brevis (ECRB). At the level of the elbow, the radial nerve divides into the superficial (sensory) branch and the deep branch, also known as the posterior interosseous nerve (PIN). The superficial radial sensory nerve emerges between the brachioradialis and extensor carpi radialis longus (ECRL) and travels distally towards the wrist and supplies the lateral dorsum of the hands, dorsal thumb, and dorsal proximal digits of the second to fourth digits. The PIN innervates the extensor compartment of the forearm that includes: supinator, extensor digitorum, extensor digiti minimi, extensor carpi ulnaris (ECU), abductor pollicis longus, extensor pollicis longus (EPL), extensor pollicis brevis (EPB), and extensor indicis.[5]
Etiology
The syndromes can be the result of external pressure, anatomic anomalies, as well as systemic and local factors. External forces can cause compression of the nerve as the pressure between the external surface, and the nerve can result in repeated or prolonged increases in pressure. Examples of external compression include leaning on the affected extremity or from medical equipment such as splints or casts. Anatomic factors can also contribute to the entrapment, and these can include space-occupying lesions such as lipomas, fibromas, ganglion cysts, as well as hematomas. Other local factors include the presence of osteoarthritis, rheumatoid arthritis, and gout. Finally, systemic factors can contribute to the compression of nerves, and these include obesity, chronic inflammatory states, diabetes, hypothyroidism, peripheral edema, and pregnancy. All of these factors trigger an inflammatory process that contributes to the symptoms of nerve compression.[6][7]
Epidemiology
Carpal tunnel syndrome is the commonest and most well-studied nerve compression syndrome of the hand. In the United States, it has an annual incidence of 1 to 3 persons per 1000 and a prevalence of 50 people per 1000. The incidence increases with age, affecting women more commonly than men with a ratio of 10 to 1. Activities and occupations that require a high degree of a repetitive activity involving the hand or upper extremity are more commonly associated with nerve compression syndromes. Additionally, conditions that trigger a chronic inflammatory state, such as diabetes or hypothyroidism, are also frequently implicated in the development of nerve compression.[2][8]
Pathophysiology
The consequences of prolonged or repetitive compression may result in inflammation, fibrosis, and demyelination of the affected nerve. Compressive forces are thought to result in varying degrees of microvascular damage, which range from mild compression that obstructs venous flow resulting in congestion and edema, to severe compression, which can result in arterial ischemia. Inflammatory responses result in the production of inflammatory cytokines such as interleukin-1 (IL-1), interleukin-6 (IL-6) substance P, tumor necrosis factor-alpha, bradykinin, and prostaglandins. Fibrosis can worsen the effects of mechanical compression, as it prevents the natural sliding and gliding of the nerve. Lastly, demyelination can lead to abnormalities in axonal signaling. A combination of these factors listed may produce axonal degeneration, which results in a poorer prognosis and more prolonged recovery.[1][2]
According to Seddon, nerve injury can subdivide into three classifications: neuropraxia, axonotmesis, and neurotmesis. Neuropraxia is the earliest and mildest form of nerve injury in which there is only myelin sheath injury or ischemia. The axon and surrounding connective tissue are undamaged. In electrodiagnostic studies, this could demonstrate conduction slowing or block. In axonotmesis, there is an injury to both axons and the myelin sheaths with sparing of the surrounding connective tissue. This damage results in Wallerian degeneration with subsequent axonal regrowth. Prognosis with axonotmesis is variable and depends largely on the distance of the nerve to the target muscles. In the most severe form of nerve injury, neurotmesis is the complete disruption of the axon and supporting structures, without the possibility of axonal regrowth. In comparing the degrees of severity of these injuries, remyelination may take a matter of weeks, while axons regenerate at a rate of approximately 1 mm per day.[9]
History and Physical
Nerve compression syndromes produce a variety of signs and symptoms, depending on the nerve that is affected. For all of these syndromes, it is essential to obtain a detailed history from each patient regarding the nature and duration of their symptoms. A patient’s medical history is another crucial component to consider as the presence of systemic disease can be contributing to the symptoms experienced. These can include chronic inflammatory conditions (autoimmune disease, diabetes, cardiovascular disease, osteoarthritis), pregnancy, obesity, and hypothyroidism. In performing a physical exam, pay attention to any anatomic factors that can result in nerve compressions such as the presence of masses (lipomas, fibromas, ganglion cysts) or hematomas. It is also essential to assess the entire affected extremity and the cervical spine to ascertain if the lesion is occurring proximal to the hand. More detailed signs and symptoms for each syndrome are listed below based on the affected nerve.[1][2][7][8]
Median Nerve
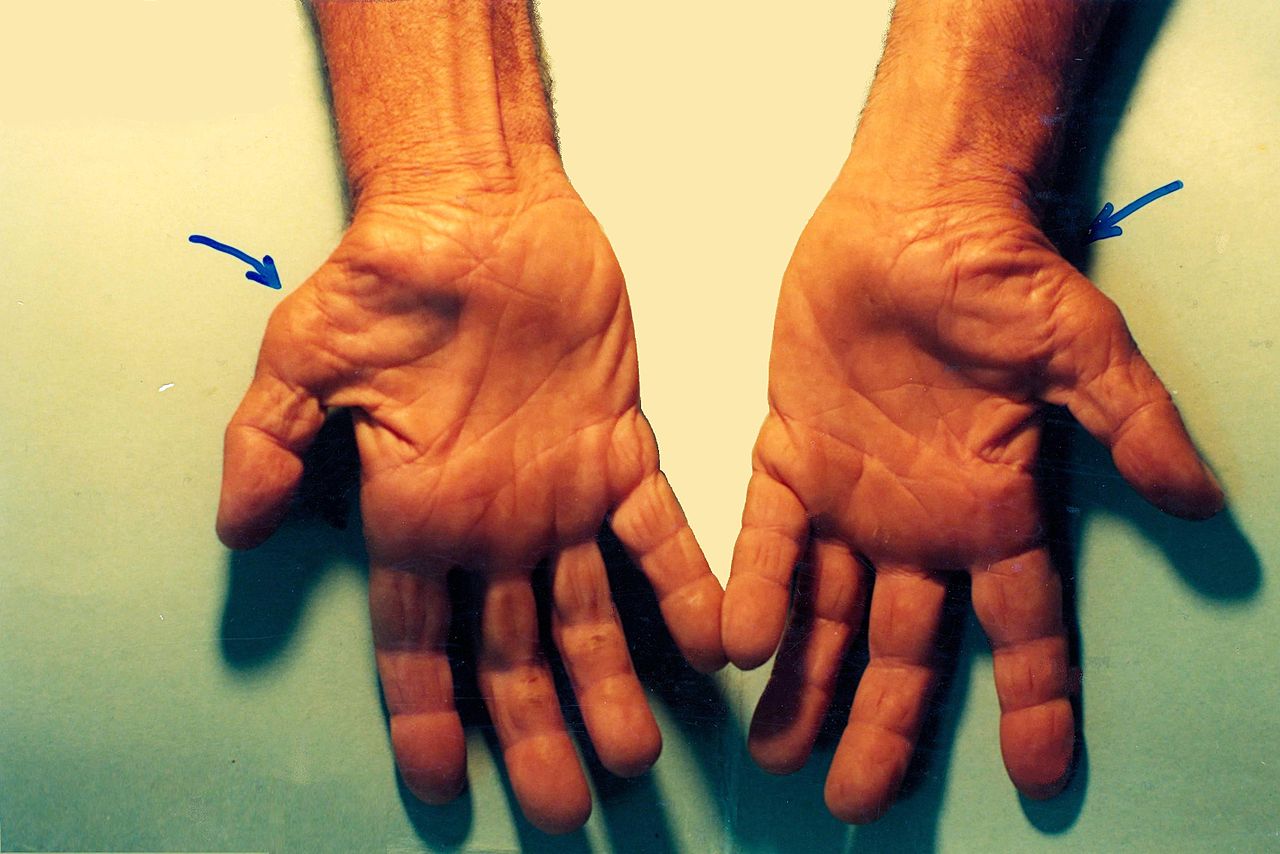
Median nerve compression at the hand and wrist is called carpal tunnel syndrome. It is the most common type of nerve injury and results from compression of the median nerve at the wrist as it passes between the carpal bones and the flexor retinaculum. It is typically the result of repetitive use of the hands but can be the result of other factors such as obesity, diabetes, pregnancy, and hypothyroidism. Patients often report numbness, tingling, and pain that worsens at night. These symptoms can be elicited from activities that involve prolonged wrist flexion and/or extension. Shaking the hand may alleviate the symptoms. They may also be weakness and clumsiness of the hand with activities such as gripping and grasping. The physical exam is an important component in the diagnosis of carpal tunnel as it can help distinguish carpal tunnel from other diagnoses such as proximal median neuropathy (pronator syndrome) and cervical radiculopathy. Sensory symptoms involve the thumb, index, long, and lateral half of the ring finger. There will be no sensory loss at the thenar eminence with carpal tunnel syndrome when compared to more proximal lesions. If motor weakness is present, it is typically evident with weakness of thumb abduction. There may also be atrophy of the thenar eminence as the median nerve innervates many muscles in this region. Signs that will be absent in carpal tunnel syndrome include weakness in forearm pronation, finger abduction, and finger extension. There are several provocative tests used to diagnose carpal tunnel syndrome[8][10]:
- Tinel sign is performed by gently tapping on the median nerve at the carpal tunnel. A positive result is when there is an electrical shock sensation in the median nerve distribution. The sensitivity of this test is 50 percent, and the specificity is 77 percent.
- Phalen test is performed by flexing the wrist for 60 seconds. A positive result is when there is numbness or tingling in the median nerve distribution. The sensitivity of this test is 68 percent, and the specificity is 73 percent.
- Carpal tunnel compression test (Durkan test) is performed by pressing the examiner’s thumbs over the carpal tunnel and holding pressure for 30 seconds. A positive test occurs with the onset of pain or paresthesia in the median nerve distribution. The sensitivity of this test is 87 percent, and the specificity is 90 percent.
- Flick sign: History of awakening with symptoms and shaking the hand to provide relief. This sign has the maximum specificity (96%) in the diagnosis of carpal tunnel syndrome.[11]
Ulnar Nerve
Ulnar neuropathy at the hand or wrist (commonly called ulnar tunnel syndrome) can result from a variety of reasons that include ganglion formation, lipoma, tumors, carpal bone fractures, and external pressure such as the use of a screwdriver, bicycle, wheelchair, or walker. Ulnar neuropathy at this level can be the result of compression of the ulnar nerve at three zones.[1][12][13][12]
- Zone 1 compression occurs with nerve compression proximal to or within the Guyon canal, occurring before the bifurcation of the ulnar nerve into the superficial and deep branches. Because the nerve has yet to bifurcate into sensory and motor branches, compression at this site will result in both motor and sensory symptoms. The motor weakness of all the ulnar-innervated intrinsic muscles of the hand will be present along with sensory deficits over the hypothenar eminence and the small and ring fingers.
- Zone 2 compression occurs distal to the bifurcation and affects the motor branch exclusively; this will manifest with motor weakness of the ulnar innervated intrinsic muscles without any sensory deficits along the ulnar nerve distribution.
- Zone 3 compression occurs distal to the bifurcation affecting only the superficial branch of the ulnar nerve, manifesting as a sensory disturbance to the palmar aspect of the little finger and the palmar-ulnar ring finger. There will be no hypothenar and interosseous weakness.
The initial aspect of the physical exam should be to observe for hypothenar or interossei atrophy, clawing of the fingers, or inability to cross the fingers. This deficit demonstrates the weakness of the ulnar-innervated intrinsic hand muscles. Palpation of the hand and wrist may elucidate the presence of masses or tenderness that could indicate a carpal fracture. The neurological exam will help to distinguish ulnar neuropathy of the wrist from more proximal lesions. If present, sensory disturbances will be seen at the palmar small finger and ulnar half of the ring finger. There will be no sensory changes on the dorsal medial hand or medial forearm, which can present in more proximal lesions such as cubital tunnel syndrome, cervical radiculopathy, or brachial plexopathy. Motor weakness will manifest with weakness of finger abduction. There will be a weakness of distal interphalangeal (DIP) flexion, thumb abduction, of finger extension. Other findings in the physical examination can include:
- Weak grasp from loss of metacarpal-phalangeal flexion strength
- A weak pinch from loss of thumb adduction
- Froment sign: this is performed by having the patient attempt to hold a piece of paper between the thumb and index finger. The examiner then attempts to pull the paper out of the patient’s fingers. A positive result is seen with compensatory interphalangeal joint hyperflexion by the flexor pollicis longus, which is innervated by the anterior interosseous nerve. This is to compensate for the loss of thumb adduction from the weakness of the adductor pollicis longus.
- Wartenberg sign, which presents as an abduction posturing of the little finger, due to weakness of the adducting palmar interosseous muscle.
Radial nerve
Radial nerve compression at the hand and wrist typically involves the superficial branch; a condition referred to as Wartenberg syndrome or cheiralgia parestheica. This is a relatively rare condition but can be caused by local trauma to the wrist such as distal radial fractures or from external compressions, such as with handcuffs, wristwatch, or bracelets. This nerve can also become compressed by soft tissue masses such as lipomas or ganglion cysts. Symptoms are strictly sensory and there, are no motor deficits noted. Patients typically present with pain, tingling, or paresthesias along the dorsolateral aspect of the wrist, hand, and fingers. Symptoms of pain predominate over other sensory symptoms. Patients may also have the aggravation of their symptoms with motions that involve repetitive wrist flexion and ulnar deviation. Physical examination should elucidate the presence of masses or signs of external pressure. There will be no motor deficits noted nor signs of atrophy. Patients may have decreased grip strength, but this is typically secondary to pain rather than to specifically identifiable weakness. The sensory examination may demonstrate abnormal sensation to light touch and 2-point discrimination on the dorsolateral aspect of the wrist and hand. Provocative tests that can be utilized to diagnose Wartenberg’s syndrome include[1][14]:
- Tinel sign: gentle tapping over the course of the superficial branch of the radial nerve resulting in the reproduction of pain and/or paresthesias. This is the most common finding.
- Dellon test: THis test is performed with active, forceful hyperpronation of the forearm with flexion and ulnar deviation of the wrist, which reproduces symptoms of pain.
- Finkelstein test: performed by asking the patient to make a fist around the thumb and ulnar deviate the wrist. A positive test is indicative of De Quervain tenosynovitis (tendonitis of the first dorsal compartment). This test may be positive in patients with Wartenberg syndrome as the neuropathy, and first dorsal compartment tenosynovitis may coexist.
Evaluation
Multiple diagnostic modalities may be utilized to evaluate for nerve compression syndromes, and they can include[1][7][14][12]:
- Electrodiagnostic studies: Electromyography and nerve conduction studies help to localize the nerve involved as well as where along the course of the nerve it is affected. Additionally, testing can serve as a baseline for comparison with future studies during the course of treatment. It is important to note that normal electrodiagnostic studies do not rule out disease, and clinical correlation should include the patient’s history and physical examination findings.
- Plain radiographs: May be useful during instances where there is a history of trauma, or there is suspicion of a fracture. It can also help to identify cases of osteoarthritis, bony prominences or osteophytes, and the presence of orthopedic hardware that could compress nerves.
- Magnetic Resonance Imaging (MRI): Can be useful in the identification of ganglion cysts, synovial or muscular hypertrophy, edema, vascular disease, as well as nerve changes. The cross-sectional area and space available for the nerve can also be measured and compared to accepted normal values.
- Ultrasound: The use of nerve ultrasonography has increased recently. It can measure the cross-sectional area and the longitudinal diameter of the nerve. It can also identify compressive lesions. Ultrasound may also evaluate the presence of local edema. Additionally, ultrasound may help distinguish between different causes of wrist pain that can include tendonitis or osteoarthritis.
- Serologic studies: There are no blood tests used to specifically support the diagnosis of nerve compression, but the use of these tests may be necessary for medical conditions that can either promote nerve compression or can mimic their symptoms. Some of the most frequently encountered conditions include diabetes and hypothyroidism. The assessment of a patient’s fasting blood glucose, hemoglobin A1c, or thyroid function tests may be helpful in the general management of the patient. Other conditions that could mimic nerve compression include deficiency of vitamin B12 or folate, vasculitides, and fibromyalgia.
Treatment / Management
Treatment of nerve compression syndromes divides into non-surgical and surgical approaches.
Nonsurgical treatment
Most instances of nerve compression are manageable non-operatively. Initially, it is important to instruct the patient to avoid repetitive use of the affected extremity and to modify symptom provoking wrist movement. This modification can be through proper ergonomic changes to activities of daily living or work activities. Counseling on weight loss and increased aerobic activity may also be beneficial as obesity can contribute to the development of nerve compression. The use of wrist splints can be beneficial to minimize motions that provoke symptoms. Wearing wrist splints is typically recommended during night-time or symptom-provoking activity. However, the regular use of splints during the day is not recommended as it can lead to stiffness of the wrist. The recommended duration for wrist splinting varies from as little as one week to as much as twelve weeks. Non-steroidal anti-inflammatories can be beneficial to reduce inflammation and provide symptoms relief. Additionally, physical therapy or hand therapy may be beneficial as it can release myofascial restriction and help reduce edema. In refractory cases, corticosteroid injections may be an option. Corticosteroid injections are not only useful in the treatment of nerve compression syndromes but can also be used to confirm the diagnosis as well as be a useful predictor of surgical success. A single corticosteroid injection can have moderate success in treating underlying symptoms. For example, one injection improved carpal tunnel syndrome symptoms in 76% of patients after six weeks. However, the symptoms are rarely long-lasting, and only 22% remained symptom-free at 1 year.[7] It is also vital that the patient should be medically optimized and treated appropriately for medical illnesses that could compound or cause nerve compressive syndromes.[8][14][15]
Surgical treatment
Surgical decompression can be a consideration after the failure of non-operative treatment. Typically non-operative treatment is attempted for a period of at least 3 months. Surgical decompression involves the identification of the affected nerve and release of adhesions or fascial bands that are contributing to the compression. Additionally, the surgeon can excise soft tissue masses that contribute to the compression. Surgical treatment can be considered as a first-line option in the instance when there is trauma or nerve compression from orthopedic hardware.[8][14][15]
Differential Diagnosis
It is vital to consider nerve compressive syndromes that occur proximal to the level of the hand and wrist. Additionally, consideration for local anatomic factors is necessary. General considerations are listed below along with differentials specific to each syndrome discussed[8][12][15]:
Carpal Tunnel Syndrome:
- Anterior interosseous compressive neuropathy
- Flexor tendonitis
- Pronator syndrome
- Wrist osteoarthritis
General considerations:
- Brachial plexopathy
- Cervical radiculopathy or myelopathy
- Compartment syndrome
- Fibromyalgia
- Motor neuron disease, e.g., amyotrophic lateral sclerosis
- Thoracic outlet syndrome
- Vasculitis and Raynaud phenomenon
- Vitamin deficiency
Ulnar Tunnel Syndrome:
- Cubital tunnel syndrome
- Extensor carpi ulnaris tendonitis
- The hook of hamate fracture
- The triangular fibrocartilage complex (TFCC) tear
Wartenberg’s syndrome
- De Quervain tenosynovitis
- Intersection syndrome
- Lateral antebrachial cutaneous nerve neuritis
Prognosis
Carpal tunnel syndrome
The normal course of carpal tunnel syndrome is that it tends to be progressive in the vast majority of patients. However, there may be fluctuations in the severity of symptoms between weeks. Patients with mild disease will have improvement with conservative treatment. Up to 80% of patients will have an improvement in symptoms with a corticosteroid injection. For these patients, only 22% remain symptoms free after one year. However, failure to improve from an injection is a poor prognostic indicator for success from surgical intervention. With surgical intervention, the success rates range from 70 to 90% in improving or relieving symptoms of carpal tunnel syndrome following a year after surgery. The rate at which symptoms persisted following one year postoperatively ranges from 2 to 20% depending on the severity of the initial presentation.[7][16]
Ulnar tunnel syndrome
Ulnar tunnel syndrome is a rare neurological disorder with limited data available regarding treatment outcomes. Conservative management can be successful in some cases. However, the mainstay treatment for severe cases remains surgical, especially during instances where there is an identifiable mass or lesion, causing compression. The reported outcomes in case studies have yielded good outcomes, but there is a lack of large, comparative studies published.[12]
Wartenberg syndrome
Most patients experience spontaneous resolution of their symptoms, with up to 71% of patients reporting excellent outcomes from conservative management. Surgical outcomes have yielded mixed results with success rates as high as 74% in a study by Lanzetta and Foucher, whereas a report by Calfee et al. reported that 55% of patients treated operatively continued to have symptoms at 3.5-year follow-up.[14]
Complications
Complications from non-operative treatment can include the progression of symptoms, atrophy, and stiffness. Complications from surgery can include unsuccessful or incomplete surgical decompression, persistent symptoms, recurrence of symptoms, worsening of symptoms, iatrogenic nerve injury, neuroma formation, hematoma formation, and damage to surrounding structures.[7][14][17]
Deterrence and Patient Education
Nerve compression syndromes of the hand can be treated well with conservative methods. It is crucial to optimize the patient’s medical status to ensure there is no systemic cause contributing to the nerve compression symptoms. Once the cause is determined, patients should receive education regarding the modification of activities that aggravate symptoms. When conservative treatment fails, or symptoms persist, there are surgical options available for patients to pursue that can have success in treating their condition. Additionally, educational resources can be utilized by health care providers, patients, and the general public to improve outcomes. These resources include:
- American Academy of Orthopedic Surgeons
- American Academy of Neurology
Enhancing Healthcare Team Outcomes
Nerve compression of the hand is a relatively common occurrence in the general population, especially with carpal tunnel syndrome. Because of the varied presentation and difficult diagnosis, the syndromes are best managed by an interprofessional team. Clinicians need to diagnose these conditions carefully with the patient’s history and physical examination. The importance of a physical exam cannot be stressed enough as the compressive syndromes of the hand need to be distinguished from other compressive syndromes that occur more proximally in the upper extremity or the cervical spine; this will help guide the clinician to the appropriate level of compression. Additional factors that merit consideration include external sources of compression, anatomic abnormalities, and the presence of systemic disease.
Management of nerve compression syndromes typically begins with non-operative management. These modalities include rest, activity modification, use of non-steroidal anti-inflammatory drugs (NSAIDs), splinting, and physical therapy. Corticosteroid injections are another option that can be offered to patients to help improve symptoms. The majority of cases will respond well with these conservative treatments, but these compressive syndromes can be progressive and may eventually require surgical intervention. Patients need to understand that symptom reversal is gradual after treatment. Physical therapy is a useful treatment approach in many patients, but requires patience as symptom reversal and restoration of function is time-dependent. Patients with pain may need to be managed by a pain specialist and a pharmacist; the key is to avoid opioids, so the pharmacist should work collaboratively with the clinician to determine the best medication regimen for pain relief while minimizing adverse events. Nursing can monitor progress along with the clinician, provide patient counseling, and check for adverse events; in the event any red flags present, the nurse should report to the clinician and/or pharmacist as appropriate. They can also serve as a bridge or contact point to the managing clinician for any other therapists (e.g., PT, OT) on the case. This type of interprofessional team approach will provide optimal patient results while minimizing morbidity and adverse outcomes. [Level V]
There is moderate evidence to support the effectiveness of surgical intervention in specific instances. However, in some cases, recurrence may be an issue if there is no lifestyle alteration.[17][18] This is why a full-on interprofessional approach is needed for optimal outcomes. [Level 5]