Hyperoxaluria
- Article Author:
- Aniruddh Shah
- Article Editor:
- Sharanya Ramakrishnan
- Updated:
- 8/11/2020 11:35:29 AM
- For CME on this topic:
- Hyperoxaluria CME
- PubMed Link:
- Hyperoxaluria
Introduction
Renal calculi are the products of crystallization of specific stone-forming components seen in about 10% of people, and 80% of these are calcium stones.[1] The most common stone, calcium oxalate, is formed due to an imbalance between the levels of calcium and oxalate in the body. The causes of excess oxalate or hyperoxaluria can be classified based on the etiology and severity of clinical presentation, into primary (idiopathic) and secondary (metabolic) hyperoxaluria. Although they both present with kidney stones, they differ in the extent and rapidity of onset of local and systemic complications.[2][3]
The acute management of renal calculi has been studied and standardized. Still, it is imperative to understand if and when to evaluate a patient further for an underlying cause of the calculi. This will not only improve the patient's quality of life but also prevent or delay recurrences and the dreaded complications of hyperoxaluria.
Etiology
Hyperoxalurias can be broadly divided into primary and secondary based on their etiology.
Primary hyperoxaluria is caused by an inherent defect or absence of enzyme activity, ultimately leading to increased levels of oxalate in the body. Of the three types of primary hyperoxaluria, type 1 is the most common, being responsible for 80 percent of the cases.
- Primary hyperoxaluria type 1: Glyoxalate is produced as an intermediate molecule in the metabolism of hydroxyproline, glycolate, and glycine and is generally detoxified in the peroxisome of the hepatocytes by the enzyme alanine:glyoxylate-aminotransferase (AGT), which converts glyoxalate to glycine. In the event of a deficiency or absence of this enzyme, glyoxalate accumulates in the cytosol, where it is converted to oxalate by lactate dehydrogenase. A deficiency of this B6-dependent enzyme (AGT) has been linked to the AGXT gene mutation on chromosome 2.[3][4][5][6]
- Primary hyperoxaluria type 2: On chromosome 10, the gene GRHPR codes for the enzyme glyoxalate/ hydroxypyruvate reductase (GRHPR). This enzyme converts glyoxalate to glycolate. A deficiency of this enzyme will lead to a buildup of glyoxalate and eventually of oxalate.[3][6]
- Primary hyperoxaluria type 3: This is the least common type, wherein a deficiency of enzyme mitochondrial 4-hydroxy 2-oxoglutarate aldolase coded by the gene HOGA1 on chromosome 9 fails to convert 4-hydroxy 2-oxoglutarate into glyoxalate. This will divert more of the glyoxalate towards the oxalate pathway.[7][3]
Secondary hyperoxaluria mainly pertains to excess exogenous oxalate gained either through diet or due to intestinal pathologies. The following are some of the secondary causes of hyperoxaluria that should be considered while making a diagnosis:
- Diet: Sources rich in oxalate include spinach, rhubarb, beet, tea, among others. Although this seems like an obvious culprit, increased oxalate in the diet plays a small role in increasing the total urinary oxalate excretion.[8]
- Increased vitamin C is a risk factor, as vitamin C is a precursor to oxalate. Calcium combines with oxalate in the intestine, which protects against renal stones, and hence decreased calcium in the diet becomes a factor.[9][10]
- Enteric hyperoxaluria: As mentioned above, calcium in the intestine makes oxalate insoluble and prevents it from leaking into the blood and eventually into the kidney. However, intestinal pathologies leading to fat malabsorption cause a buildup of unabsorbed fatty acids and bile salts in the intestinal lumen that bind to the calcium ingested through the diet, barring it from combining with oxalate. These go further in the intestinal lumen and increase the colon's permeability to oxalate. The soluble oxalate, which could not combine with calcium diffuses passively into the blood and is filtered by the kidneys. In addition to this, vitamin B6 deficiency ensues in these conditions, which is also culpable for increased endogenous oxalate production.[11][3]
- A similar mechanism of hyperoxaluria is seen in patients post-bariatric surgery, wherein fat and calorie malabsorption are induced to reduce obesity-associated comorbidities.[11]
- Pancreatic insufficiency in patients of chronic pancreatitis also exhibits saponification due to the binding of calcium and unabsorbed fatty acids, in turn leaving unbound oxalate to be absorbed and filtered by the kidneys.[12][13]
- Oxalobacter formigenes: This gram-negative, oxalate-degrading bacteria colonizes the colon. Antibiotic use, inflammatory bowel disease (IBD), or changes in diet may lead to disruption of the colonies and buildup of oxalate.[3][11]
Epidemiology
In 2012 a survey suggested that in the United States, approximately 1 out of 11 people suffered from renal stones, which are considerably higher than the numbers recorded in a previous study done 13 years back. The same survey revealed that men had been affected more than women (10.6% men and 7.1% of women).[14]
Calcium stones comprise about 80% of all kidney stone diseases, with calcium oxalate being the far more predominant one (approximately 75%).[15][16] The risk of recurrence with calcium stone is about 60% in 10 years without the appropriate preventive measures.[15]
Primary hyperoxalurias (PH) are quite rare and may be diagnosed very late into the course of the disease, usually after the development of nephrocalcinosis or end-stage renal disease (ESRD). PH1 is the most common form of PH. The prevalence of the disease ranges from 1 to 3 per million population with an approximate incidence rate of ~1 : 100,000 live births per year in Europe. Higher rates are reported from inbred populations. PH is responsible for <1% of the pediatric ESRD population in registries from the USA, UK, and Japan.[17]
Pathophysiology
Stone formation, in general is caused by a combination of abnormal factors that influence the supersaturation and rate-controlling processes involved in the crystallization of the various stone-forming minerals. The principal thermodynamic driving force for both these phases is the degree of supersaturation of the fluid in which this initiation occurs. The laws of crystallization hold for both intracellular and extracellular crystallization. The following steps lead to the formation of oxalate stones:
-
Nucleation: This is the first step leading to crystallization and can occur either homogeneously or heterogeneously. Homogeneous nucleation requires a greater supersaturation of calcium oxalate as compared to the solvent. By contrast, heterogeneous nucleation requires lesser amounts of precipitating salts due to the presence of proteins and other organic polymers that act as receptacles with chemically active cell surfaces, making this the most likely mechanism of nucleation.
-
Supersaturation: An RSS level is used to measure the supersaturation level and varies with each solute. The level at which nucleation occurs is referred to as the formation product of the mineral concerned. This provides a range of supersaturation values that are capable of facilitating de novo crystal nucleation.
-
Crystal growth and agglomeration: Once a crystal is established, the surrounding urine facilitates the growth of this crystal. These growing crystals stagnate at sites where the urinary flow is relatively sluggish, either due to narrowing of the tubule as in the proximal tubule, when it meets the loop of Henle or at the papillary base where renal tubules bend, or at the slitlike openings of the collecting duct that structurally favor plugging. When these come in contact with the surrounding epithelial cells, these grow into macromolecules. These travel along the length of the tubules whilst continuously accumulating crystals on the way.
- Rate of crystal growth: This is determined by the RSS as well as accompanying components that promote crystallization like magnesium, citrate, pyrophosphate, matrix substance A, various uncharacterized urinary proteins, and glycoproteins, and the polymerized form of Tamm–Horsfall protein amongst others.[18]
Histopathology
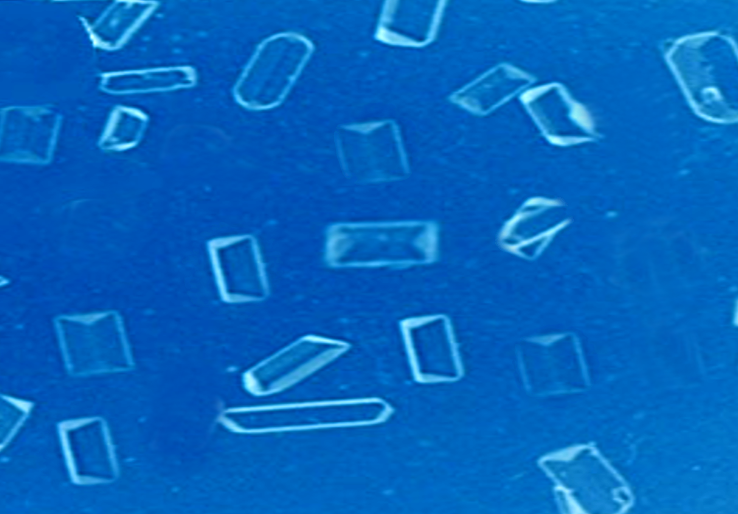
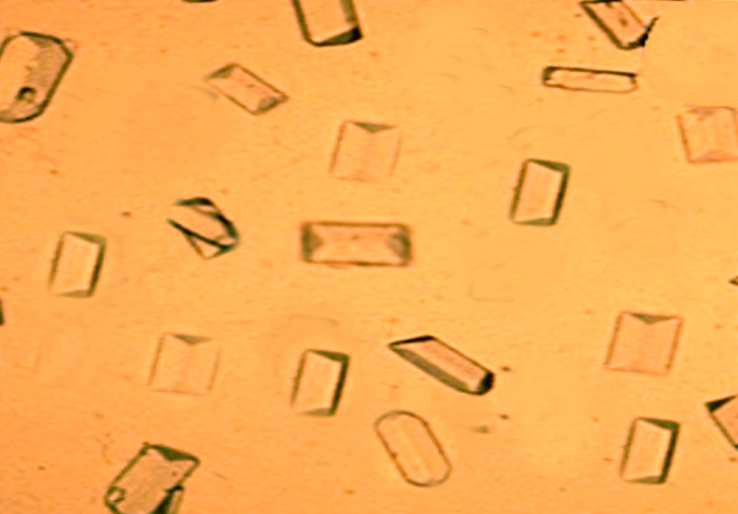
Gross appearance: The gross appearance is unique to each type of stone. An oxalate stone reveals a nodular surface of the stone on gross examination. On dissection, the stone reveals concentric laminations and radial striations.
Microscopy: Examination of the individual crystals reveals thin and plate-like structures that generally acquire a 'pyramidal' or 'dumb-bell' shape through twinning, as seen in urinary sediments, based on the etiology of the hyperoxaluria.[18]
History and Physical
The clinical presentation of both primary and secondary hyperoxaluria can be seen in several age groups and are preferably studied as renal and systemic manifestations. However, secondary hyperoxaluria patients have a lower propensity for systemic manifestations.
Renal: The renal manifestations can be seen mainly due to the increased urinary oxalate excretion and its combination with calcium, leading to nephrolithiasis or urolithiasis, depending on the location of their deposition. We would expect a patient to come in with symptoms of renal stones like abdominal or flank pain radiating to the groin along with nausea and vomiting. This is also commonly associated with burning micturition and hematuria.
The crystallization and deposition of calcium oxalate within the renal tissues are known as nephrocalcinosis. Together these two processes of nephrocalcinosis and stone formation will cause inflammation and renal injury that eventually will lead to a decline in renal function and ESRD.[4] A GFR below 30–50 mL/min per 1.73 m2 will exacerbate this condition by further decreasing the oxalate excretion in urine and increasing its buildup in the plasma leading to deposition within secondary tissues. This threshold level is known as the supersaturation point of calcium oxalate, exceeding which the crystallization and deposition begin.[17]
Primary hyperoxaluria type 2 is milder as compared to type 1, mainly due to lesser urinary oxalate excretion, whereas recurrent renal stones are characteristic of primary hyperoxaluria type 3.
Systemic: The secondary deposition of calcium oxalate, more commonly associated with primary hyperoxaluria, gives rise to various systemic manifestations. Based on the organ affected, these include:
- Heart: Conduction defects, heart blocks, and cardiomyopathy.
- Skeletal: Bone pain, pathological fractures, the involvement of the joints like synovitis, and chondrocalcinosis.
- Nervous system: Peripheral neuropathy, retinopathy, and cerebral infarcts.
- Vascular: Non-healing ulcers and gangrene due to ischemia of blood vessels. There have also been reports of refractory hypotension.
- Hematology: Anemia due to oxalate deposition in the bone marrow that is unresponsive to erythropoietin-stimulating agents (ESA).[19][20][17]
Evaluation
A patient presenting with symptoms of renal calculi should be investigated with a urinalysis, KUB, and, if needed, a CT scan of the abdomen and pelvis. The evaluation of the underlying hyperoxaluria should be considered separately.
The diagnosis of primary hyperoxaluria is rare and more often than not, becomes a differential only after the development of ESRD.[3][7] A few circumstances which could be cues for investigating this condition are:
- An episode of renal calculi in a child.
- Recurring episodes of renal calculi in adults.
- A patient diagnosed with nephrocalcinosis, which is the deposition of calcium oxalate within the renal tissue. If this nephrocalcinosis is accompanied by a decrease in GFR, it is even more suggestive of primary hyperoxaluria.
- A patient diagnosed with renal failure but without a clear underlying cause to it or with a history of renal calculi.
- If the renal stones sampled from the patient are indicative of primary hyperoxaluria type 1, which are the whewellite stones.[21]
After establishing the diagnosis, an analysis of the stone should be done and the morphology should be studied for determining the possible etiology. Calcium oxalate monohydrate and calcium oxalate dihydrate assume dumbbell and pyramid forms, respectively. This may help us further in determining the exact cause as the dumbbell-shaped calcium oxalate monohydrate crystals (whewellite) are seen in primary hyperoxaluria, whereas mixed stones (whewellite and weddellite) are seen in secondary hyperoxaluria.[3][4][17]
Any patient suspected of hyperoxaluria should be tested for urinary oxalate excretion after collecting a 24-hour urine sample and correcting it for the patient's body surface area. Normally the urine oxalate excretion is <0.45 mmol/1.73 m² per 24 hours. Urinary oxalate excretion of more than 1.0 mmol/1.73 m2 per 24 hours is typically seen in primary hyperoxaluria. To differentiate between type 1 and 2 biochemically, glycolate and glycerate levels are useful if elevated. Raised urinary glycolate level is seen in primary hyperoxaluria type 1 and glycerate in type 2.[3][17][4] For type 3, an increased urinary level of 4-hydroxyoxoglutarate (HOG), and dihydroxyglutarate (DHG) are suggestive.[7]
A urine oxalate: creatinine ratio is also widely used, although the age-specific normal limits should be used for comparison.[22][17][3]
An associated kidney injury is common in cases of primary hyperoxaluria, which might lead to a decrease in GFR, which would, in turn, lead to a decrease in oxalate excretion. These measurements would be misleading, and hence a plasma oxalate concentration would be needed in these situations. A normal plasma oxalate level is between 1-5 μmol/L.[3][17]
Confirmatory tests include measuring the AGT enzyme activity following a liver biopsy or directly analyzing the suspected genes for mutations. Non-invasive definitive diagnosis of primary hyperoxaluria is provided by testing of AGXT, GRHPR, and HOGA1 genes.[3]
Treatment / Management
Management of a patient with hyperoxaluria includes conservative, medical, and surgical measures along with the treatment of nephrolithiasis. Isolated treatment of the renal stone could be conservative using fluids and alpha-blockers or surgical if the case is complicated by urosepsis. Here we focus on the prevention and treatment of patients with an established diagnosis of hyperoxalurias.
Fluid Intake: An increased fluid intake increases the volume of urine and hence prevents the supersaturation of calcium oxalate. An intake of more than 2 L/day has been recommended to decrease the intratubular oxalate deposition.[1][20][23]
Urinary Alkalinization: To keep the urinary pH at a favorable 6.2-6.8, urinary alkalinization could be achieved with potassium citrate (0.1-0.15 g/kg body weight) or sodium citrate in cases of renal failure. This prevents the crystallization of calcium oxalate stones. This is usually given along with thiazide diuretics to decrease the calciuria.[24][17][3] The use of orthophosphate has proven to reduce the formation of calcium oxalate stones.[25]
Dietary Measures: These measures do not play a major role in primary hyperoxaluria as the excess oxalate in this condition is endogenous. However, dietary modifications in secondary hyperoxaluria have been useful. Although randomized controlled trials have proven that restricting calcium in the diet is detrimental, the beneficial effect of calcium supplementation in protecting against oxalate stones is still unclear.[20][1] The intake of vitamin C should be restricted.[26][20] Consumption of oxalate-rich foods like tea, dark-leafy vegetables, rhubarb, and chocolates should be limited.[1][9] Enteric hyperoxaluria patients are also advised on a low-fat diet along with the restriction of oxalate-rich foods.[11] Higher sodium levels in the diet lead to an increased calciuria and thus an increased propensity to form stones. Limiting the amount of sodium intake has proven to prevent recurrences of renal stones.[27][1] An excess protein in the diet increases the calcium excretion in the urine and should be restricted in patients with a history of renal stones.[28][1]
Pyridoxine Supplementation: In the pathophysiology of primary hyperoxaluria type 1, we discussed the role of the defective AGT enzyme leading to hyperoxaluria. This enzyme requires pyridoxal phosphate as a cofactor. Patients with a deficiency of this enzyme could be supplemented with 5-20 mg/kg pyridoxine to boost the activity of the AGT enzyme. The genotype of the patient's enzyme will determine the responsiveness to pyridoxine, and a positive response has been seen in only 30% of the patients. Type 2 and type 3 primary hyperoxalurias will not benefit from this as their defective enzymes do not have pyridoxine as a cofactor.[4][17][29]
Oxalobacter formigenes, although involved in the pathogenesis of hyperoxaluria when deficient, confers limited benefit when given as an oral supplement.[22][30]
Dialysis: The role of dialysis is controversial. Oxalate levels of 30-45 μmol/L lead to tissue deposition, and the aim of dialysis is to keep the oxalate level below that to prevent supersaturation. In patients with ESRD due to primary hyperoxaluria, dialysis can not sufficiently remove oxalate as quickly as it accumulates in the blood. In these cases, a special intensive dialysis regimen has to be put into place, which has more sessions per week as compared to the standard dialysis therapy, along with combining both hemodialysis and peritoneal dialysis to achieve maximum oxalate clearance.[3][4] Due to these drawbacks, dialysis has limited indications. Patients waiting for a liver or renal transplants, post-transplant patients with suboptimal hepatic or renal function, or elderly patients who are unfit for surgery are a few of the circumstances where dialysis is considered.[17]
Transplant: The procedures to choose from are an isolated liver transplant, isolated renal transplant, or combined liver-renal transplant. The final decision is made after due consideration of various factors. Patients with ESRD or a GFR approaching ESRD require renal transplants. A liver transplant is the only curative measure in patients of type 1 primary hyperoxaluria as the AGT enzyme is the culprit here. In patients that show a positive response to pyridoxine by increasing AGT activity, an isolated renal transplant may be considered.[31] However, the results of isolated renal transplants have been dismal due to lower allograft survival rates in patients of primary hyperoxaluria. Children and young adults may be considered for a sequential liver-renal transplant.[3]
Primary hyperoxaluria type 2 manifests due to the defective enzyme glyoxalate/ hydroxypyruvate reductase (GRHPR), which can be found in tissues other than the liver hence an isolated renal transplant has been recommended for these patients.[32]
Primary hyperoxaluria type 3 patients are not usually referred for renal transplantation as the chances of them developing ESRD are quite rare, whereas the data for renal transplantation in patients of secondary hyperoxaluria is scarce.[3][22]
Differential Diagnosis
The differential diagnosis should include conditions that lead to nephrolithiasis, specifically calcium oxalate stones, and to excess deposition in tissues leading to nephrocalcinosis.
- Primary hyperoxaluria type 1
- Primary hyperoxaluria type 2
- Primary hyperoxaluria type 3
- Idiopathic calcium oxalate stone disease. This condition presents as a milder form of primary hyperoxaluria along with mild calciuria.
- Secondary hyperoxaluria
- Nephrocalcinosis of prematurity[4]
Prognosis
The prognosis of hyperoxaluria depends on the type of hyperoxaluria, time of diagnosis, and early initiation of treatment, amongst other things. Type 1 reportedly has the worst prognosis, with very high possibilities of the patient developing nephrocalcinosis or ESRD.[3] The availability of intensive dialysis, along with medical treatment, can slow down the progression of the disease.[33] However, if the blood oxalate level can not be kept in check, an organ transplant is the only available cure.[34]
Studies suggest that most enteric hyperoxalurias have a better prognosis if medical interventions coupled with dietary measures are followed strictly. The patient is maintained on a low oxalate diet continuously while being treated for his underlying condition. However, in an event where ESRD does occur in these patients, careful monitoring of peritransplantation and post-transplantation oxalate along with adequate urine output is done, which does not show any unfavorable parameters.[35]
Complications
The complications of calcium oxalate stones in the urinary tract include:
- Urosepsis
- Hydronephrosis
- Anuria
- Formation of abscesses
- Urine extravasation
- Post-renal obstruction and the gradual decline of kidney function[18]
The complications of hyperoxaluria were also discussed as part of its clinical features. The development of nephrocalcinosis, further leading to ESRD, is the most dangerous complication. Along with the kidneys, the involvement of the other organ systems is also seen as a complication of the long-term buildup of oxalate in the blood.
Deterrence and Patient Education
Patient education in individuals with a tendency for oxalate precipitation is of utmost importance to reduce episodes of stone formation. Preventing dehydration by consuming adequate water as well as restricting oxalate-rich food in the diet are two of the easiest ways to prevent recurrent stone formation. A decreased vitamin C in the diet is advisable. Sodium and protein content in the diet should be decreased along with oxalate.[1][9] Additionally, patients with secondary hyperoxaluria or an underlying etiology for steatorrhea, are kept on a low-fat diet to prevent the entrapment of calcium ions in this luminal fat, and subsequent increase in absorption of oxalate.[36]
The inheritance occurs in an autosomal recessive pattern, and genetic counseling is thus important in patients with primary hyperoxaluria.[4]
Enhancing Healthcare Team Outcomes
Patients suffering from renal calculi due to hyperoxaluria should be made aware of all the different preventive measures they should adhere to, to avoid recurrent stone formation. The diagnosis and counseling are the responsibility of both the physician and the nurse practitioner. The dietician is responsible for carefully crafting their dietary chart whilst keeping in mind their dietary restrictions on oxalate-rich food, along with an increase in fluid intake. Patients with recurrent episodes and children with early-onset nephrolithiasis are examples of patient groups that would understandably require a high level of motivation to follow the strict preventive measures. If followed diligently, it could decrease the recurrence of stones in patients. This would be a combined effort of physicians, nursing staff, and dieticians.[37]
A good nephrolithiasis preventive program should be combined with regular evaluation for systemic symptoms and monitoring of kidney and liver functions. A specialist can be brought in for cases refractory to dietary measures and drug therapy. For curative interventions like liver-kidney transplants, a surgeon should be consulted. The role of alkali citrate to reduce urinary calcium oxalate saturation and precipitation has been studied, and the long-term administration to patients of hyperoxaluria has been advised to reduce the recurrence of calcium oxalate stones. [24]
Collaboration shared decision making and communication are key elements for a good outcome. The interprofessional care provided to the patient must use an integrated care pathway combined with an evidence-based approach to planning and evaluation of all joint activities. [Level 2]