Ciliary Dysfunction
- Article Author:
- Brittany Stern
- Article Editor:
- Girish Sharma
- Updated:
- 7/17/2020 7:13:02 AM
- For CME on this topic:
- Ciliary Dysfunction CME
- PubMed Link:
- Ciliary Dysfunction
Introduction
Primary ciliary dyskinesia (PCD), formerly known as immotile cilia syndrome, is a disorder of motile cilia structure and function that results in chronic oto-sinopulmonary disease. Primary ciliary dyskinesia typically presents with respiratory distress in infants, early onset year-round cough, and nasal congestion. Primary ciliary dyskinesia diagnosis is challenging given lack of a single diagnostic test and the multitude of conditions that result in similar symptoms. Kartagener syndrome is a triad of chronic sinusitis, bronchiectasis, and situs inversus resulting from ciliary dyskinesia.[1][2][3][4]
Etiology
Primary ciliary dyskinesia is a genetic disorder with abnormal ciliary ultrastructure and movement. There are currently 33 known genes associated with primary ciliary dyskinesia, with the majority following autosomal recessive inheritance. DNAI1 and DNAH5, which encode for components of the outer dynein arm complex in cilia, are the two most common genes associated with PCD. Two rare X-linked genes, RPGR and OFD1, have also been identified.
Epidemiology
Reported prevalence is approximately 1:15,000 live births. Given difficulties in diagnosing primary ciliary dyskinesia, misdiagnosis is common, and therefore primary ciliary dyskinesia may be more prevalent than reported.
Pathophysiology
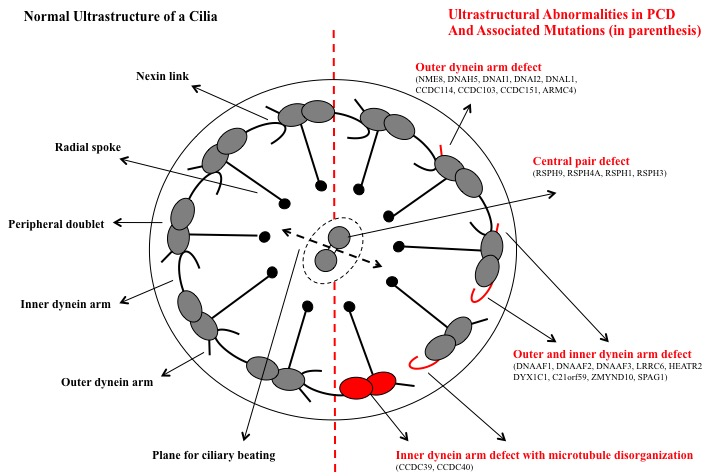
Cilia are hair-like attachments on epithelial cell surfaces of the cells lining the nasopharynx, lower respiratory tract, paranasal sinuses, middle ear, and reproductive tract. In primary ciliary dyskinesia, mutations in genes encoding for axonemal structures of motile cilia result in cilia that are abnormal in structure and function. Defects in primary ciliary dyskinesia can include outer dynein arm defects, inner dynein arm defects, central microtubular abnormalities, radial spoke defects and outer ultrastructural abnormalities (Figure). In the respiratory tract, abnormal ciliary movements cause impaired mucociliary clearance that results in recurrent and chronic oto-sinopulmonary infections. The sperm tail and fimbriae of fallopian tubes also have motile cilia. Therefore male and female infertility often occurs in primary ciliary dyskinesia patients. Situs abnormalities are also common as the defect in motile cilia during embryogenesis results in abnormal thoracoabdominal orientation. Situs inversus occurs in 50% of cases of primary ciliary dyskinesia, since normal ciliary movement needed for the visceral rotation, in the absence of normal ciliary function during embryogenesis, becomes a chance occurrence. Kartagener syndrome is a triad of chronic sinusitis, bronchiectasis, and situs inversus resulting from ciliary dyskinesia.[5][6][7][8]
History and Physical
The majority of newborns with primary ciliary dyskinesia develop neonatal respiratory distress with atelectasis on chest radiographs. Unlike other causes of respiratory distress in newborns, which occurs in the first few hours after birth, the respiratory distress with primary ciliary dyskinesia patients occurs 12 to 24 hours after birth in term infants. Four main clinical features have been described in primary ciliary dyskinesia including unexplained neonatal respiratory distress in term infants, early onset year-round wet cough, early onset year-round nasal congestion, and laterality defects. If three or more of these features are met, the specificity for primary ciliary dyskinesia diagnosis is more than 96%. About half of patients with primary ciliary dyskinesia also have situs inversus totalis, including transposition of the right and left lung. Male infertility is common and occurs in nearly 100% of males. Females can present with reduced fertility or ectopic pregnancy due to abnormal fallopian tube transit of oocytes. Patients may also have chronic sinusitis and nasal congestion that does not change with seasons and does not resolve between viral infections. Persistent daily cough that begins in infancy is reported in nearly 100% of primary ciliary dyskinesia patients.
Evaluation
Diagnosing primary ciliary dyskinesia is challenging as it results from a variety of defects in cilia and no single diagnostic test can detect all defects (Figure). Diagnosis is typically achieved with a combination of clinical features as described above in conjunction with diagnostic testing which may include procedures such as nasal or bronchial brush biopsy to demonstrate abnormal ciliary ultrastructure and ciliary motility. In patients older than 5 years, nasal nitric oxide measurements are sensitive and specific for diagnosing primary ciliary dyskinesia. Paranasal sinus epithelium produces nitric oxide, and nasal nitric oxide measurements are typically extremely low (less than 100 nL/min) in primary ciliary dyskinesia patients. Decreased nasal nitric oxide values can also be seen in acute viral respiratory infections and patients with cystic fibrosis. Therefore, patients should be fully recovered from viral illnesses, and cystic fibrosis should be ruled out before performing this test. Since there some of the features overlap with other chronic diseases such as cystic fibrosis, sweat chloride testing and if indicated genetic testing for cystic fibrosis should be completed in all patients being evaluated for primary ciliary dyskinesia. However, otitis media a typical feature in a patient with primary ciliary dyskinesia is not a feature of cystic fibrosis. Other conditions, such as immunodeficiency, asthma, chronic pulmonary aspiration and allergic rhinitis can also present similarly and should be considered. High-speed video microscopy analysis for the evaluation of ciliary motility is extremely sensitive and specific. Additionally, various ciliary defects can be observed on electron microscopic examination of the samples obtained by ciliary biopsy, including immotile cilia, poor motility, and ineffective and slow beat patterns. Although electron microscopy (EM) analysis can aid in diagnosis, there is a subset of patients with primary ciliary dyskinesia with no demonstrable ultra-structural abnormalities identified. Furthermore, transient ciliary dyskinesia may be acquired following epithelial injury from viral respiratory tract infections or exposure to pollutants. Genetic testing should also be considered as 33 genes have been associated with primary ciliary dyskinesia. However, not all of them are included in the commercially available primary ciliary dyskinesia genetic testing panel. In the United States, a combination of ciliary biopsy to demonstrate ciliary ultrastructural abnormalities in combination with genetic testing is used for the diagnosis, while a combination of genetic testing and high-speed video microscopy is used in the Europe.[9][10][11][12]
Treatment / Management
Primary ciliary dyskinesia patients should be managed in centers that specialize in primary ciliary dyskinesia and chronic pulmonary diseases. Primary ciliary dyskinesia patients should be evaluated by a pulmonologist two to four times per year with spirometry and surveillance cultures of sputum. A chest radiograph should be completed at the time of diagnosis and during acute respiratory exacerbations. At least once after diagnosis, chest CT to evaluate for bronchiectasis should be considered. Additionally, children with primary ciliary dyskinesia should be evaluated by ear-nose-throat specialist one to two times per year and as needed in adults. An audiology assessment should be performed since there is a high incidence of recurrent otitis media with chronic middle ear effusions and associated complications of conductive hearing loss. Patients should be monitored for chronic rhinosinusitis and nasal polyps. Primary ciliary dyskinesia patients should receive daily chest physiotherapy, influenza vaccination, and pneumococcal vaccination as indicated. Antibiotics should be prescribed for acute respiratory exacerbations. In cases with recurrent respiratory infections, long-term oral or nebulized antibiotics can be considered. Gene editing has been proposed as a possible future treatment for primary ciliary dyskinesia. Ex vivo gene repair by site-specific recombination has been found to rescue ciliary beating, which could have tremendous application in primary ciliary dyskinesia patients.
Differential Diagnosis
- Cystic Fibrosis
- Foreign body aspiration
- Idiopathic interstitial pneumonia
- Idiopathic nasal polyposis
- Immunosuppression
- Malignancy
- Post-infectious bronchiectasis
- Severe atopy
- Tracheobronchomegaly
Enhancing Healthcare Team Outcomes
Primary ciliary dyskinesia is managed by an interprofessional team of healthcare workers. Primary ciliary dyskinesia patients should be managed in centers that specialize in primary ciliary dyskinesia and chronic pulmonary diseases. Primary ciliary dyskinesia patients should be evaluated by a pulmonologist two to four times per year with spirometry and surveillance cultures of sputum. Additionally, children with primary ciliary dyskinesia should be evaluated by ear-nose-throat specialist one to two times per year and as needed in adults. An audiology assessment should be performed since there is a high incidence of recurrent otitis media with chronic middle ear effusions and associated complications of conductive hearing loss. The primary care provider and nurse practitioner should monitor for chronic rhinosinusitis and nasal polyps. These patients should receive daily chest physiotherapy, influenza vaccination, and pneumococcal vaccination as indicated. Antibiotics should be prescribed for acute respiratory exacerbations.[13][14]