Leukemia
- Article Author:
- Varun Lyengar
- Article Editor:
- Alex Shimanovsky
- Updated:
- 8/10/2020 4:42:08 PM
- For CME on this topic:
- Leukemia CME
- PubMed Link:
- Leukemia
Introduction
Leukemias are a group of hematologic disorders characterized by the dysfunctional proliferation and development of leukocytes. They can be classified as either acute or chronic according to the degree of cell differentiation and as myelocytic or lymphocytic according to the predominant type of cell involved. Many genetic and environmental risk factors have been identified, though the exact cause of most leukemia subtypes is unknown. Symptoms are nonspecific and can include fever, fatigue, weight loss, bone pain, bruising, or bleeding. Definitive diagnoses often require bone marrow biopsy, the results of which inform interprofessional treatment ranging from chemotherapy to stem cell transplantation. Prognosis is variable depending on the leukemia subtype in question.
Classification
- Acute vs. chronic: Acute leukemias are characterized by abnormal cells that are less mature, develop quickly, and leave the bone marrow as dysfunctional cells called “blasts.” These blasts crowd out healthy cells in the bone marrow, causing the rapid onset of symptoms. Blasts normally make up 1% to 5% of marrow cells, and having more than 20% blasts in the bone marrow is required for a diagnosis of acute leukemia. In contrast, chronic leukemias develop slowly and may take years to develop symptoms. They are composed primarily of more mature and functional cells, and there are generally not elevated numbers of blasts.[1][2][3]
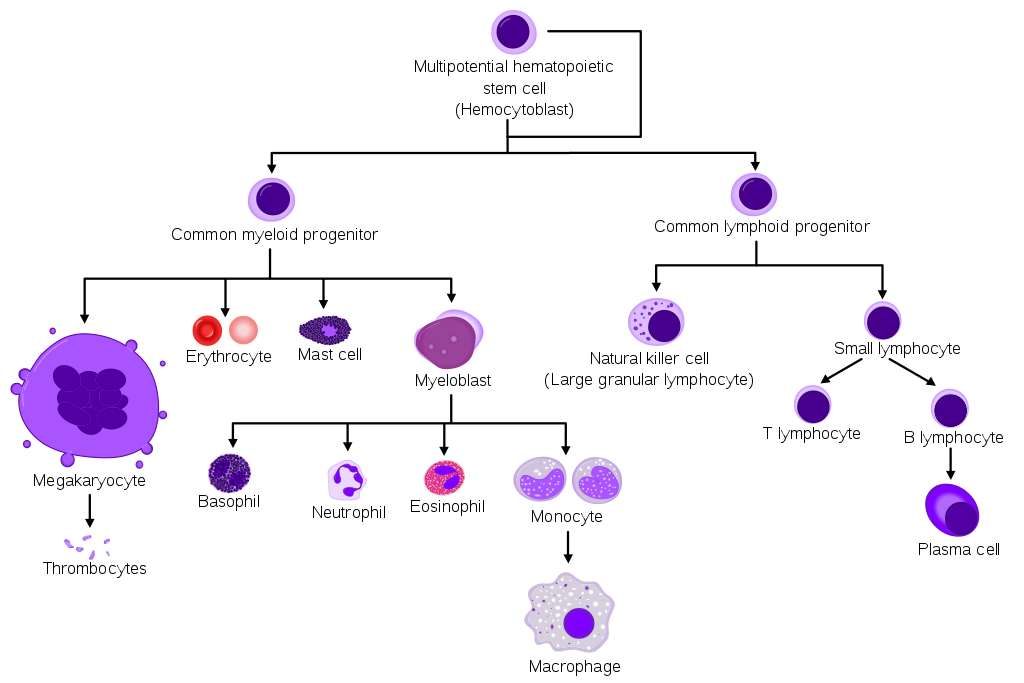
- Myeloid vs. lymphoid: Hematopoietic stem cells give rise to two types of blood cells: myeloid and lymphoid. Myeloid cells include monocytes, macrophages, neutrophils, basophils, eosinophils, erythrocytes, and megakaryocytes. Lymphoid cells include T cells, B cells, and natural killer cells.[1][2]
As such, the four major subtypes of leukemia are:
- Acute lymphoblastic leukemia (ALL): ALL occurs when primitive white blood cells of lymphoid origin reproduce without developing into normal B and T cells. It is the most common leukemia in pediatrics, accounting for up to 80% of cases in this group vs. 20% of cases in adults.
- Acute myelogenous leukemia (AML): AML is also characterized by the hyperplasia of blasts, but in this case, of myeloid origin. It accounts for half of the leukemia cases diagnosed in teenagers and people in their 20s. It is the most common acute leukemia in adults.
- Chronic lymphocytic leukemia (CLL): CLL occurs when mature but abnormal white blood cells of lymphoid origin undergo hyperplasia, leading to a monoclonal population of dysfunctional lymphocytes. Most cases occur in people between ages 60 and 70.
- Chronic myelogenous leukemia (CML): A monoclonal population of self-renewing, dysfunctional myeloid cells (e.g., neutrophils, basophils, eosinophils, macrophages) characterizes CML. Most cases occur in people between ages 25 and 60.[1][2][4][5]
Etiology
Several risk factors are associated with a higher risk of developing leukemia:
- Exposure to ionizing radiation is associated with an increased risk of multiple subtypes of leukemia.[6][7]
- Exposure to benzene is a risk factor for leukemia in adults, particularly AML.[8]
- Previous exposure to chemotherapy, especially alkylating agents and topoisomerase inhibitors, increases the risk for acute leukemia later in life.[6][7]
- A history of any hematologic malignancy is a risk factor for subsequently developing another subtype of leukemia.[9]
- Viral infections (e.g., human T-cell leukemia virus, Epstein Barr virus) are linked with subtypes of ALL.[10]
- Several genetic syndromes (e.g., Down syndrome, Fanconi anemia, Bloom syndrome, Li-Fraumeni syndrome) are associated with an increased risk of AML and ALL.[11]
Epidemiology
Leukemia is the 15th most commonly diagnosed cancer and 11th leading cause of cancer death worldwide. The disease burden is higher among males relative to females (incidence rate at 6.1 per 100,000 vs. 4.3 per 100,000), as is the death rate (4.2 vs. 2.8 per 100,000 in males vs. females).
The age distribution of chronic leukemia is unimodal; incidence and mortality rates tend to increase with age. ALL and AML, which are important diseases in both childhood and adulthood, have bimodal age distributions. Globally, the total number of leukemia cases increased by 26% from 2005 to 2015, and population growth and aging accounted for all but 3% of this.[6]
In the United States, leukemia is the 10th most common cancer and the seventh leading cause of cancer mortality. Since 2006, the incidence of the disease has increased by an average of 0.6% per year, while the mortality has decreased by an annual average of 1.5%.[5][6]
Pathophysiology
Leukemia occurs due to malignant transformation of pluripotent (i.e., can give rise to both myeloid and lymphoid precursors) hematopoietic stem cells. Rarely it can also involve a more committed stem cell that has a limited self-renewal capacity. In acute leukemias, these malignant cells are generally immature, poorly differentiated, abnormal leukocytes (blasts) that can either be lymphoblasts or myeloblasts. These blasts can undergo clonal expansion and proliferation, leading to replacement and interference of the development and the function of normal blood products with malignant cells, leading to clinical symptoms.
Acute Leukemia
In ALL, chromosomal translocation or abnormal chromosome numbers can lead to mutations in precursor lymphoid cells leading to lymphoblasts. Common mutations include t(12;21) and t(9;22), also known as the Philadelphia chromosome. In AML, chromosomal translocations, rearrangements, and gain or loss of chromosomes can lead to mutations and abnormal production of myeloblasts. One important translocation is t(15;17), which leads to the fusion of retinoic acid receptor alpha (RARA) and a promyelocytic leukemia transcription factor (PML). This leads to the development of acute promyelocytic leukemia, which can be treated with retinoic acid.
Chronic Leukemia
Chromosomal abnormalities in hematopoietic stem cells that are precursors to leucocytes are the most common cause of chronic leukemia. Examples of abnormalities are deletions, translocations, or extra-chromosomes. In CML, mutations mostly affect granulocytes (most commonly the t(9;22) translocation), and in CLL, they mostly affect lymphocytes (especially B lymphocytes). Unlike acute leukemia, in chronic leukemia, cells are partially mature. These partially mature cells do not function effectively and divide too quickly. They accumulate in the peripheral blood and lymphoid organs. This can lead to anemia and thrombocytopenia, and leukopenia.
Histopathology
Acute Leukemia
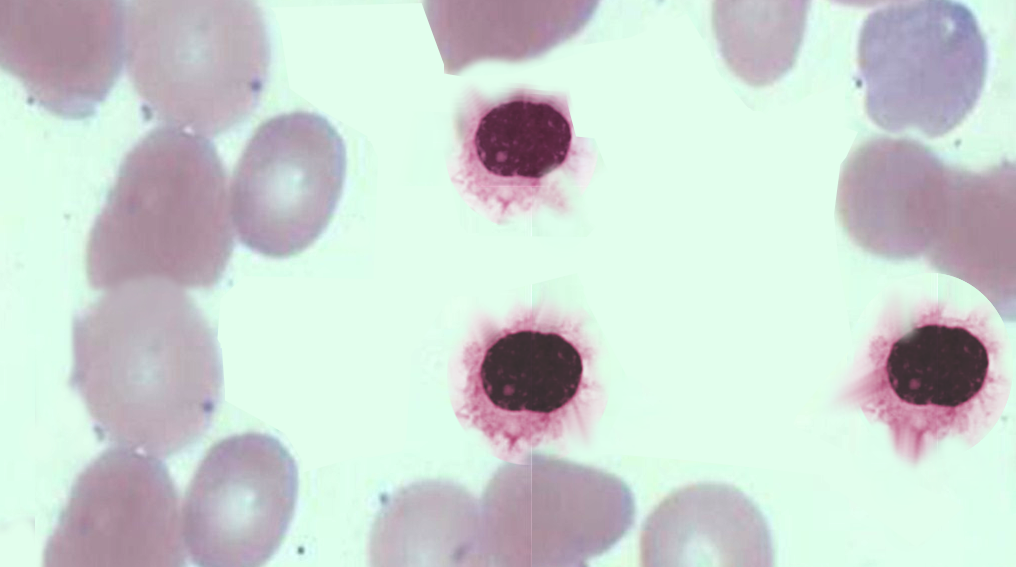
In acute leukemia, the peripheral blood smear is characterized by less than 20% blasts. It is important to obtain the bone marrow biopsy and aspirate to help differentiate between the types of acute leukemia.
In AML, there is increased bone marrow cellularity, composed mostly of granulocytic or monocytic cells with a variable number of erythroid precursors. The immunohistochemistry is generally positive for common myeloid immunostains: CD13, CD14, CD15, CD33, CD36, CD61, and CD64.
In ALL, there is also increase bone marrow cellularity, composed of B and T lymphoblasts (which have small nucleoli, dispersed chromatin, and cleaved and irregular nuclei with undetectable cytoplasm). Common T-cell lymphoid immunostains include: TdT, CD2, CD3 (cytoplasmic), CD5, CD7. Common B-cell lymphoid immunostains include: HLA-DR, CD10, CD19, CD22, CD79a (cytoplasmic), PAX5, CD20
Chronic Leukemia
In chronic leukemia, the white blood cell count is often elevated, and the diagnosis of CML/CLL can be made by looking at the peripheral blood smear. In CML, the peripheral blood usually shows greater than 100,000 white cells characterized by (i) neutrophilia; (ii) an increase in metamyelocytes, myelocytes, basophilia, eosinophilia, and monocytes (though the monocyte fraction is typically less than 3%); and (iii) a low LAP score. In CML, the translocation t(9;22) can be diagnostic by FISH on peripheral blood. Bone marrow biopsy is not necessary for diagnosis, but if done, it will show a 100% cellular marrow with increased granulocyte precursors, basophils, eosinophils, and occasional monocytes.
In CLL, the white cell count is elevated, with mostly CD5+, CD23+ B-lymphocytes. The absolute lymphocyte count (ALC) has to be > 5,000 for diagnosis. If the ALC is less than 5,000, the entity is termed monoclonal B cell lymphocytosis of undetermined significance.
History and Physical
Acute Leukemia
Acute leukemia tends to present non-specifically, although the most common presenting features include fever, lethargy, and bleeding. Hepatosplenomegaly, lymphadenopathy and musculoskeletal symptoms (especially in the spine and long bones) can also be clues to the diagnosis. Adults may also have more prominent anemia-related symptoms, such as shortness of breath, or symptoms related to thrombocytopenia, such as excessive bruising or heavy menstrual cycles.
Chronic Leukemia
Chronic leukemia subtypes occur almost exclusively in adults. Many patients are asymptomatic at the time of diagnosis, identified only incidentally after marked leukocytosis is discovered on a CBC performed for another reason. Hepatosplenomegaly and lymphadenopathy can be appreciated in some cases while bleeding and bruising are less common, presenting features relative to acute leukemia subtypes.[12]
Evaluation
The workup of leukemia is time-consuming. Multiple tests are needed to confirm a diagnosis, and subsequently, to stage the disease. Helpful initial studies include a complete blood count (CBC), complete metabolic panel (CMP), liver function test (LFT), and coagulation panel, which are often followed by a peripheral blood smear and a bone marrow specimen.
On rare occasions, leukemia can be diagnosed on histology alone. For example, AML is characterized by the presence of Auer rods (red staining, needle-like bodies seen in the cytoplasm of myeloblasts) on a peripheral smear. In most other cases, more detailed analyses – e.g., flow cytometry, cytogenetic testing – are required to distinguish between subtypes.[13]
A bone marrow aspiration and biopsy are often required for the diagnosis of acute leukemias. For chronic leukemias, peripheral blood evaluation is often enough, and an invasive bone marrow biopsy may not be needed. For example, CML can be diagnosed by looking for BCR-ABL fusion protein on peripheral blood FISH analysis, and CLL can be diagnosed by looking for a monoclonal B-cell population by doing a peripheral blood flow cytometry.
Treatment / Management
Patients with leukemia should be referred to a hematologist-oncologist to initiate treatment. Therapy varies significantly based on the leukemia subtype and patient factors (e.g., age, comorbid conditions). Chemotherapy is a mainstay of most regimens; however, radiation therapy, monoclonal antibodies, and stem cell transplantation are also options.
Notably, during induction chemotherapy for acute leukemias, supportive treatment is very important. Given that chemotherapy depletes normal blood cells, there is an increased risk of infection and bleeding, and as such, antimicrobial agents and blood-production transfusion may be required. Antibiotics, anti-fungal, anti-nausea medication, and transfusion of blood products are all to support the patient during induction vaccines against the flu and pneumonia are also recommended.
For CML in particular, it is important to acknowledge that tyrosine kinase inhibitors (i.e., imatinib) have revolutionized our treatment. CML is a consequence of an abnormal fusion gene that codes for a tyrosine kinase, an enzyme that activates signal transduction and can cause uncontrolled cellular proliferation. By blocking this signal, tyrosine kinase inhibitors maintain long-term control of the disease with fewer adverse effects relative to chemotherapy.[14]
Other forms of treatment are specific to leukemia subtypes. Recently, chimeric antigen receptor (CAR) T-cell therapy has been approved for ALL. In cases of indolent leukemia (e.g., CLL), doctors may adopt a more conservative approach (“watchful waiting”) and not actively treat slower-growing cancers. Targeted therapy for other subtypes of leukemia may be an option if an actionable mutation exists (i.e., IDH1/IDH2 mutations in AML).
Differential Diagnosis
Given that leukemia itself is a broad diagnosis with non-specific symptoms, the differential diagnosis is broad. One must rule out infection, drug effects, vitamin/micronutrient deficiencies, and other myelodysplastic disorders that can cause abnormalities in blood cell lines.
Consider the following when seeing abnormalities in the blood count:
- B12 and folate deficiencies
- Copper deficiencies
- Viral Infections (e.g., HIV, Cytomegalovirus, Epstein Barr virus)
- Drugs (chemotherapeutic agents, valproic acid, ganciclovir, mycophenolate mofetil)
- Autoimmune conditions (e.g., systemic lupus erythematosus)
Prognosis
Long-term survival with leukemia varies tremendously based on leukemia subtype, cytogenetic and molecular findings, patient age, and comorbid conditions. Broadly, the 5-year cancer survival rate for leukemia has increased from 33% in 1975 to 59% in 2005.[5]
Complications
Tumor lysis syndrome (TLS)
TLS is a complication of chemotherapy that can result when tumor cells die quickly. The widespread cellular destruction releases intracellular contents into the bloodstream overwhelming the kidneys, resulting in dangerously high serum levels of potassium, phosphorus, uric acid, and blood urea nitrogen.[15]
Disseminated intravascular coagulation (DIC)
DIC is a complication of leukemia itself in which the proteins that control blood clotting become overactive, leading to both thrombosis and hemorrhage. DIC is often associated with acute promyelocytic leukemia but can be seen in other subtypes of leukemia as well.[13]
Infection
Immunosuppression from chemotherapy, stem cell transplantation, or leukemia itself increases the risk of dangerous infections. Fever with neutropenia in an immunosuppressed patient should prompt an immediate evaluation for infection source and the initiation of broad-spectrum antibiotic therapy.[16]
Cancer
Survivors of leukemia are at an increased risk of subsequent cancers. For example, the Childhood Cancer Survivor Study demonstrated that the 30-year cumulative incidence of any cancer after leukemia was 5.6%; the median time to occurrence of the subsequent cancer was nine years. The most common second neoplasms in childhood leukemia survivors are different subtypes of leukemia or lymphoma.[9]
Enhancing Healthcare Team Outcomes
Acute and chronic leukemias are a heterogeneous group of hematologic diseases with complex diagnostic and therapeutic requirements that require an interdisciplinary team. The involvement of healthcare professionals from across specialties and disciplines - physicians, nurses, physical therapists, nutritionists, etc. - is needed to achieve effective management, mitigate adverse events, and ensure quality of life. Patient-centered communication and shared decision-making are integral to successful patient outcomes.
(Click Image to Enlarge)
(Click Image to Enlarge)
Contributed by Shabir Bhimji, MD