
Cutaneous Melanoacanthoma
- Article Author:
- Philip Cohen
- Article Editor:
- Patrick Zito
- Updated:
- 9/29/2020 4:16:52 PM
- For CME on this topic:
- Cutaneous Melanoacanthoma CME
- PubMed Link:
- Cutaneous Melanoacanthoma
Introduction
Cutaneous melanoacanthoma is a benign skin tumor. It was originally described by Bloch in 1927 as a non-nevoid type I melano-epithelioma; subsequently, in 1960, Mishima and Pinkus coined the term melanoacanthoma. The lesion morphology mimics that of melanoma. It typically presents as a solitary black nodule that has been present for nearly 6 years and has increased to a diameter of 2 or more centimeters. It is most commonly located on either the head and neck or the trunk. Microscopic examination shows an epithelial lesion in which the epidermis has a thickening of not only the stratum corneum (hyperkeratosis) but also the other layers (acanthosis); undulation of the epithelium (papillomatosis) may also be present. Importantly, throughout all layers of the entire epidermis, there is a mixture of cell types: melanocytes with large dendritic processes and keratinocytes. The pathogenesis of a melanoacanthoma remains to be established; many researchers consider it to be a variant of seborrheic keratosis. Possible etiologic factors in the development of a melanoacanthoma include secondary colonization of melanocytes, irritation-induced maturation of basal cells into squamous cells that block melanin transfer from the melanocytes into the keratinocytes, and trauma. Visual inspection is the initial evaluation of a patient with a solitary pigmented lesion that is either new-onset or older and increases in growth; typically, a melanoacanthoma is not considered as a diagnostic possibility since the lesion has many clinical, dermatoscopic and reflectance confocal microscopy features that may also be observed in melanoma. The diagnosis is usually established after a biopsy of the lesion has been performed; Fontana Masson stain or an S100 or melan-A—also known as melanoma antigen recognized by T-cells 1 (MART-1)--immunoperoxidase stain can confirm the presence of melanocytes at all levels of the epidermis. Conservative excision of melanoacanthoma is the treatment of choice since incomplete removal of the tumor can result in persistence and continued growth or recurrence of the lesion. It is important for the individuals who provide primary care to patients with new or changing solitary pigmented lesions to refer the patient to a clinician who can evaluate them and perform a biopsy. Management following the biopsy consists of routine wound care and periodic follow-up evaluation to ensure that the melanoacanthoma does not recur.
Melanoacanthoma is a rare benign neoplasm. It can occur in the mouth (or other mucosal epithelium); in this location, it is referred to as an oral melanoacanthoma.[1] In contrast, it can also occur on non-mucosal sites and is designated as a cutaneous melanoacanthoma.[2][3][4][5][6][7] This paper focuses on non-mucosal melanoacanthoma; henceforth, unless otherwise specified, melanoacanthoma refers to cutaneous melanoacanthoma.
Benign non-nevoid melanoepithelioma of the skin was described in German by Dr. B. Bloch in the Archives of Dermatology and Syphilology in 1927. He classified the lesion into two types:
- Type I melanoepithelioma (currently referred to as a melanoacanthoma)
- Type II melano-epithelioma (now considered to be the pigmented variant of a seborrheic keratosis).[2][8][9]
More than 30 years later, in 1960, Mishima and Pinkus presented 13 new cases of benign pigmented skin tumors that were characterized by a proliferation of dendritic melanocytes mixed with hyperplastic epidermal cells (which they described as basal and prickle type Malpighian cells). They considered the lesions to be identical with the type I benign non-nevoid melanoepithelioma that Bloch had described. They also proposed that the tumor be designated as a melanoacanthoma.[2]
After they studied seborrheic keratoses and melanoacanthomas in 1969, the Sanchez Yus and Simon Huarte changed their histologic diagnosis of melanoacanthoma to that of melanocytic seborrheic keratosis.[3][7] In 1991, they suggested that dermatopathological vocabulary eliminates the term melanoacanthoma.[7] However, neither their proposed nomenclature to replace melanoacanthoma nor their recommendation to abolish the term has not been accepted.
Melanoacanthoma typically presents as a solitary lesion on either the head and neck or the trunk of an elderly individual older than 60 years of age. It is usually a slowly growing black or hyperpigmented cutaneous asymptomatic tumor. The appearance shares many common features with those of a melanoma; therefore, a biopsy is typically done to exclude the possibility of cancer. Once the diagnosis is established, excision is often performed if residual melanoacanthoma is present. Recurrence is rare after the benign tumor has been removed.
Etiology
The etiology of melanoacanthoma has not been definitively established. Many researchers consider the lesion to be a variant of seborrheic keratosis. However, the unique pathologic features of a melanoacanthoma, the presence of melanocytes throughout all the layers of the epidermis, are not universally noted in seborrheic keratoses.
The secondary colonization by dendritic melanocytes of nonmelanocytic lesions has been observed. This phenomenon has been observed in reactive (such as lichen simplex chronicus), benign (such as verruca), and malignant (such as squamous cell carcinoma) lesions. Therefore, it has been postulated that dendritic melanocyte colonization may account for the presence of these pigmented cells that are found at all levels of the epidermis in a melanoacanthoma.
Irritation of a seborrheic keratosis may be associated with the development of a melanoacanthoma. Yet, exposure of a seborrheic keratosis to either croton oil or surgical trauma or both did not result in the subsequent development of the pathological features observed in a melanoacanthoma. However, some investigators postulate that irritation may promote the maturation of the basal cells of the epidermis into squamous cells that would subsequently impede the transfer of melanin from the melanocytes into the keratinocytes.[2][10]
The solitary melanoacanthoma, in some patients, has been observed to be located in areas of trauma. The extent to which trauma directly or indirectly influences the potential development of a melanoacanthoma is unknown. However, several patients with oral melanoacanthomas associated either the onset or persistence of their lesions to continued exposure of a traumatic etiology or the resolution of the mucosal lesion to the cessation of the irritating factor.[1] Also, a few individuals who have a cutaneous melanoacanthoma relate the onset or progression of the lesion with a traumatic insult to their skin.
Mishima and Pinkus described a 68-year-old Japanese man with a 4.5 x 2.8 centimeter dark brown melanoacanthoma on the nape of his neck. The lesion had been present for 10 years. However, during the prior 2 months, the melanoacanthoma displayed rapid growth after trauma to the area had occurred.[2]
Mishra et al. reported a 61-year-old woman whose melanoacanthoma presented on the cutaneous medial half of her right lower eyelid (sparing the punctum) as a painless 8 x 4 x 3 millimeter brownish-black firm mobile tumor. The pruritic lesion had been noted 4 years earlier. However, it slowly began to increase in size after tree extract had fallen into her right eye.[11]
Jain et al. published the case of a 58-year-old woman with multiple melanoacanthomas of 10 years duration. One of the lesions was a 4 x 3 cm ulcerated plaque on her mid-back. The authors attribute the large size of the melanoacanthoma to have caused the ulceration by several mechanisms: compromised blood flow, friction, pressure while lying down, and trauma. However, it is reasonable to postulate that ulceration (perhaps secondary to friction or pressure or both) resulted in an originally smaller progressively growing into a much larger tumor.[9]
Epidemiology
Melanoacanthoma is considered by many investigators to be a rare variant of seborrheic keratosis. Indeed, nearly all of the published literature on melanoacanthoma is included in this paper, and the total number of cases is only 140. Therefore, to attempt to establish the incidence of this pigmented lesion, studies evaluating the number of melanoacanthomas compared to either other specimens evaluated or other variants of seborrheic keratoses diagnosed have to be used. However, based on variance observed in these studies, the incidence of melanoacanthoma can not be definitively established since it ranges from 1 in 100,000 pathology specimens to between approximately 1% to 38% of seborrheic keratoses.[2][3][4][5][6][7][8][9][10][11][12][13][14][15][16]
Prince et al. described 5 patients with melanoacanthoma. They considered the lesion to be rare since 500,000 consecutive skin biopsy specimens were required to diagnose the 5 cases. Hence, their observed incidence of melanoacanthoma was 1 case per 100,000 specimens.[4]
Mishima and Pincus, in their seminal paper introducing the term melanoacanthoma, reported 13 patients. They commented that these cases were derived from more than 400 pigmented skin tumors; they had not included nevi in the group of 400 lesions. Hence, their incidence of melanoacanthoma, with regards to other pigmented lesions, was only 3% (13 of 400).[2]
Roh et al., in 2016, cited two previous studies from the Korean literature and added their retrospective investigation regarding the incidence of melanoacanthoma. One of the earlier studies, by Ahn et al., observed only one melanoacanthoma in the 127 seborrheic keratoses they reviewed for 6 years, a 0.8% incidence. Similarly, the other prior investigation by Lee et al. also noted a low incidence (1%) of melanoacanthoma; only one lesion was found in the 101 seborrheic keratoses they studied. However, Roh et al. found a much higher incidence (9.2%) of melanoacanthoma; they observed 19 lesions in the 206 seborrheic keratoses they reviewed.[6]
A group of Spanish researchers also discovered much higher incidences of melanoacanthoma in their serial studies. Initially, in 1969, they reported their evaluation of the number of dendritic melanocytes in the basal and suprabasal layers of seborrheic keratoses; they found that 38% of the seborrheic keratoses (8 of 21 lesions) demonstrated features consistent with melanoacanthoma.[3] Subsequently, between 1968 and they noted that 53 of 189 consecutive cases of seborrheic keratoses (28%) were melanoacanthoma. They further extended their studies during the next 3 years but only noted 14 of 161 seborrheic keratoses (8.7%) to be melanoacanthoma.[7]
Melanoacanthoma is a lesion that typically appears in older individuals without a predilection for either gender. Retrospective studies and several case reports of patients with melanoacanthoma have been published. To get a better understanding of the epidemiology of this lesion, the individual study results shall be combined with the information pooled from the individual reports to allow for the salient features of melanoacanthoma to be discerned.
Gender has been provided for 52 melanoacanthoma patients. This includes 28 men and 24 women. Hence, the ratio of men to women is 1.2 to 1.
The specific ages of the patients at the diagnosis of melanoacanthoma were provided for 20 men and 20 women. The men ranged in age from 40 years to 85 years; the median age and the mean age of diagnosis were both 63 years. The age range was wider in women, yet the median and mean age of diagnosis were younger. The women ranged in age from 27 years to 84 years; however, the median age of diagnosis was 59 years, and the mean age was nearly a decade younger at 55 years.
In summary, melanoacanthoma usually presents when patients enter their seventh decade. When the onset age for the men and the women were considered together, the diagnosis age ranged from 27 years to 85 years. The age at diagnosis had a median of 61 years and a mean of 60 years.
Melanoacanthoma occurs worldwide. Patient reports originate from many countries including Belgium,[16] Brazil,[12] Canada,[10] India,[8][9][11] Italy,[15] Japan,[2] South Korea,[6] Spain,[3][7] and the United States of America.[2][5][13][14]
The patient’s race or nationality was described for 43 individuals. Melanoacanthoma has been most often described in Whites: 18 patients (42%).[2][4][10][13][15] Spanish (8 patients),[3] Indian (6 patients),[8][9][11] Asian (4 patients: 3 Japanese[2][4] and 1 Korean) and Black (4 patients)[14] were next most frequent races. The lesion has only been observed a single person from each of the following races: Brazilian,[12] Haitian-Creole,[14], and Italian.
Pathophysiology
The pathophysiology of melanoacanthoma remains to be determined. Many researchers postulate that melanoacanthoma is a type of seborrheic keratosis. However, clinical investigations and observations suggest that melanoacanthoma is not merely an irritated seborrheic keratosis. Trauma-induced cutaneous injury and partial inhibition of melanin pigment transfer from large melanocytes with numerous dendritic processes to adjacent keratinocytes may also have a role in the pathophysiology of melanoacanthoma.
Mevorah and Mishima, in 1965, attempted to establish whether irritation with 20% to 50% croton oil or trauma from excision of half of the lesion would induce a seborrheic keratosis to develop pathologic changes observed in a melanoacanthoma. They observed an increase in the number of keratinocytes in the stratum spinosum of the seborrheic keratoses. However, in contrast to melanoacanthomas in which the melanocytes are dispersed throughout the epidermis, the melanocytes of the irritated and traumatized seborrheic keratoses predominantly remained in the epidermal basal layers at the junction between the epidermis and dermis.[17]
More recently, Gutierrez et al. described an 85-year-old man who not only presented with a large melanoacanthoma on his abdomen but also two biopsy-confirmed pigmented seborrheic keratoses on his posterior shoulder and axilla. The concurrent presence of multiple pigmented seborrheic keratoses and a solitary melanoacanthoma prompted the clinicians to hypothesize that the melanoacanthoma pathophysiology might be different than the etiology of the seborrheic keratoses in that individual.[14]
Melanoacanthomas tend to develop in sites of prior trauma. Hence, it has been postulated that cutaneous injury may be an etiologic factor in the pathophysiology of melanoacanthomas. Additional investigation is needed to establish whether trauma-associated skin injury directly or indirectly influences the development of melanoacanthoma.
An incomplete block in melanin transfer, from melanocytes to keratinocytes, has been demonstrated in the electron microscopy studies performed on a melanoacanthoma from the back of a 74-year-old man. The ultrastructural evaluation of the lesion confirmed the presence of keratinocytes and richly dendritic, unusually large, melanocytes; the majority of melanin pigment was found in the latter cells whose features resembled those that have been noted in inflamed skin from African Americans in whom melanin transfer from melanocytes to keratinocytes is blocked. The investigators hypothesized that changes in the rate and pattern of keratinocyte differentiation resulted in an alteration of the cell’s surface and thereby inhibited or prevented the transfer of pigment from the melanocytes.[2][10]
Histopathology
A melanoacanthoma is an epithelial lesion. Staining with hematoxylin and eosin stain shows not only shows thickening of the stratum corneum (hyperkeratosis), but also the thickening of the entire epidermis (acanthosis); this corresponds to the raised papule, plaque or nodule morphology of the lesion. Cellular atypia of the keratinocytes is absent. The undulation of the epidermis (papillomatosis) may also be present; this corresponds to the verrucous surface that is observed on some of the lesions. Throughout all layers of the epidermis, there is hyperpigmentation; this results from melanocytes with pronounced dendrites that can be found not only in the lower layers but also in the upper layers of the epidermis. Keratin-filled pseudocysts may also be present in the epidermis. In the underlying dermis, perivascular lymphocytic inflammation may be present.[4][10]
Some researchers consider melanoacanthoma to have two histologic variants. The first variant, the diffuse subtype, has melanocytes unevenly scattered throughout the entire epidermis. The second variant, the clonal subtype, shows melanocytes and keratinocytes, each individually clustered into small nests within the epidermis.[4][9][11]
Dopa and Fontana-Masson silver stains can highlight melanin and melanocytes. Therefore, they can be used to distinguish a melanoacanthoma from seborrheic keratosis. In a melanoacanthoma, both of these stains highlight melanin and melanocytes will show positive staining throughout all layers of the epidermis. However, in seborrheic keratosis, the positive staining is only found in the lower layers of the epidermis.[3][7][10]
A unique patient with a clear cell melanoacanthoma was reported by Pierard in 1986. He described a 47-year-old patient (whose gender was not stated) who presented with a black globular tumor of a few years duration that was located on the anterior area of the leg. The clinical diagnosis entertained was a nodular melanoma. Microscopic examination showed an epithelial lesion consisting of two distinct compartments. One compartment, associated with the pilosebaceous appendages, consisted of normal epidermal cells. The second compartment consisted of a mixture of large glycogen-filled pale keratinocytes practically devoid of melanin and large dendritic melanocytes; the latter cells were abundantly packed with melanin and distributed throughout all layers of the epidermis. Correlation of the pathologic findings and the clinical presentation established the diagnosis of a clear cell melanoacanthoma; no recurrence was observed during 3 years of follow-up after the lesion was excised.[16]
Immunoperoxidase staining may help determine whether a lesion is a melanoacanthoma when the diagnosis is suspected. Cytokeratin stains, such as cytokeratin 7 (CK7), stain keratinocytes positive. Therefore, which shows positive staining of the keratinocytes in a melanoacanthoma will stain positive with CK7. Hence, immunoperoxidase staining for cytokeratin can be used to assist in differentiating melanoacanthoma from melanoma.[13]
Melanocytic markers S100 and melan-A, also known as melanoma antigen recognized by T-cells (MART-1), will stain melanocytes in melanoma, benign nevi, and also melanoacanthoma. Therefore, to establish the diagnosis of melanoacanthoma, other features noted on hematoxylin and eosin staining are important.[13]
If a brown chromagen is used to identify the positive-staining cells when immunoperoxidase stains are performed, it may be difficult to differentiate the brown staining melanocytes from the brown appearing melanin-laden keratinocytes. However, if a red chromagen is used, this problem is resolved since only the melanocytes will show the red staining.
Electron microscopy not only confirms the presence of highly dendritic melanocytes were present but also reveals that the transfer of melanin from these melanocytes to the adjacent keratinocytes was defective. Also, except in the basal layer, Langerhans cells were present throughout the epidermis.[2][10]
History and Physical
Melanoacanthoma is acquired, almost always solitary, pigmented lesions of the skin that appear in adulthood. In most patients, they are asymptomatic and progressively enlarge slowly.[9][10][11] [14] However, albeit uncommon, they can grow rapidly, itch, be tender, or become irritated.[2][14][12][13]
The duration that the melanoacanthoma was present prior to biopsy-confirmation of the diagnosis was provided for 29 individuals. It ranged from 5 weeks to 40 years. The investigators for two of the individuals provided descriptive durations: "as long as he could remember" in a 40-year-old man with multiple melanoacanthomas on his left upper eyelid and "since childhood" in a 56-year-old woman with a melanoacanthoma on her abdomen.[2][4][8][9][10][11][13][14][15][16]
The skin lesion was present for more than 10 years in 28% (8 of 29) of the patients, and between 6 to 10 years for 24% (7 of 29) of the patients. For 35% (11 of 29) of the patients, it was only present for less than 3 years. Overall, the median duration of the melanoacanthoma was present before establishing its diagnosis was 6 years.
The size of the melanoacanthoma was described for 36 patients. The greatest diameter ranged from minute to 2 millimeters to 15 centimeters. Five of the lesions (14%) were greater than or equal to 10 centimeters; they were characterized as being 'giant.'[2][4][8][9] Eleven of the lesions (31%) were between 2.5 centimeters to 9.9 centimeters; they were referred to as 'large.'[2][4][5][10][14] A third of the melanoacanthoma was between 1 to 2 centimeters, and only 22% (8 of 36) were less than 1 centimeter. The median size was 2 centimeters.
The color of the melanoacanthoma can mimic that of melanoma; it was specified for 28 patients. Nine of the patients' melanoacanthoma were composed of 2 colors. In addition, it was described as either pigmented or hyperpigmented for an additional 8 individuals.[4][9]
Half of the lesions (14 of 28) were black.[2][13][14][16] The next most common colors were either both black and brown (21%, 6 of 28) or brown (18%, 5 of 28).[2][4][8][10][11][12] The melanoacanthoma was either black and gray (7%, 2 of 28) or blue and brown (4%, 1 of 28) in the remaining patients.[2][4]
The melanoacanthoma color in one study was not able to accurately be interpreted based on the information provided. The 8 melanoacanthomas evaluated had either 1, 2, or more colors. The most common colors were either light brown (5 patients) or dark brown (4 patients); black, blue-gray, and pink melanoacanthoma were each observed in 1 patient.[5]
The appearance of a melanoacanthoma often mimics that of melanoma.[2][3][8][9][10][12][13][14][16] It typically presents as a solitary papule, nodule, or plaque.[4][8][10][12][13][14] However, its surface can be lobulated or verrucous.[2][4][14][8][13][15]
The melanoacanthoma location was specified for 52 patients. The head and neck (46%, 24 of 52) or the trunk (30%, 16 of 52) were the most common sites. Most of the remaining 24% of melanoacanthomas were located either on an extremity (12%, 6 of 52) or the genitals (6%, 3 of 52: either inguinal or penis or scrotum in 1 patient) or the buttock and hip (4%, 2 of 52).
The head and neck melanoacanthoma were most commonly located on the scalp (28%, 6 of 21). Other sites included the ear (19%, 4 of 21), the eyelid (19%, 4 of 21), the face (19%, 3 of 21 on the preauricular area and 1 of 21 on the nose) and the neck (15%, 3 of 21). The specific location on the head and neck was not stated in 3 patients.
A 58-year-old woman had multiple slowly growing melanoacanthomas of 10-years duration (2%, 1 of 52). Her lesions were located on the neck, trunk (chest, abdomen, and back), extremities (proximal arms and legs), and buttocks. Several of her lesions (particularly those on her left buttock and periumbilical region) were horn-like cutaneous nodules. Also, one of the melanoacanthomas on her trunk became ulcerated.[9]
Jain et al. also summarized the features of another patient (described by Shenoy et al.) with multiple genital and perianal melanoacanthomas; the man's lesions were located not only on his trunk (lower abdomen) and extremity (left inner thigh) but also on his penile shaft and perianal skin. Multiple melanoacanthomas were also reported on the left upper eyelid of a 40-year-old man. In contrast to patients with cutaneous melanoacanthoma in whom multiple tumors are seldom observed, multiple lesions are not uncommon in individuals with oral melanoacanthoma.[1][9]
Rarely, a melanoacanthoma presents as a cutaneous horn, mimicking a verruca or a squamous cell carcinoma. The buttock and periumbilical lesions on the woman with multiple melanoacanthomas morphologically appeared as cutaneous horns. Also, the melanoacanthoma on the left upper eyelid of a 45-year-old man developed into a horn-like cutaneous nodule that grew downwards and extended to below his lower eyelid.[9]
Evaluation
Biopsy, partial or excisional, is the most appropriate approach for the evaluation of a lesion in which the diagnosis of melanoacanthoma is being considered. An adequate biopsy specimen should be obtained so that the pathologist has sufficient tissue to examine and thereby be able to provide an accurate diagnosis.
The clinical features of a melanoacanthoma mimic those of melanoma. Therefore, the clinician often cannot establish the diagnosis of melanoacanthoma based solely on the lesion's morphologic appearance. Thus, the evaluation of a suspected melanoacanthoma may include dermoscopy and reflectance confocal microscopy. However, biopsy for microscopic examination is usually necessary to establish the diagnosis.
Dermoscopy
Dermoscopy is a non-invasive in vivo examination technique to evaluate the surface of the skin. Subsurface skin structure in the epidermis can be visualized using a hand-held dermatoscope. Dermoscopy, most often used to evaluate pigmented skin lesions, has been done by four groups of investigators to study melanoacanthoma.
Dermoscopic features of melanoacanthoma, which mimicked those of a pigmented Spitz nevus, were initially described by Rossiello et al. in 2006. They examined a raised 4 millimeter verrucous pigmented left hip lesion and viewed a starburst pattern consisting of symmetrically distributed pigmented streaks on the periphery; this pattern has previously been considered diagnostic for a pigmented Spitz nevus. Also, in the center, they noted other features of a pigmented Spitz nevus, which can also occasionally be found in a melanoma: (1) gray to whitish hyperkeratotic areas and (2) multiple, black to brown, dots and (3) multiple globules.[15]
Shankar et al., in 2010, evaluated a giant 10 cm by 5 cm melanoacanthoma. Dermoscopic examination showed a regular pigmentary network. A cribriform pattern of ridges and fissures characteristic for a seborrheic keratosis was also observed. There were no melanoma dermoscopic features.[8]
Subsequently, in 2015, Chung et al. studied the lesions from eight patients with melanoacanthomas with dermoscopy. They noted features of seborrheic keratoses (comedo-like openings, hairpin vessels, milia-like cysts, moth-eaten border, and sharp demarcations) in all of the lesions. However, they also observed features specific for melanoma features (atypical dots, blue-white veil, granularity, and polymorphous vessels) on six of the lesions.[15]
More recently, in 2016, Shahriari et al.'s dermoscopic examination of melanoacanthoma which presented as a 7 mm black papule on the left submandibular area showed a lesion with a verrucous contour, scale, and features that prompted the investigators to consider a pigmented nodular melanoma: irregular blue-white and black clods.[13]
Reflectance Confocal Microscopy
Reflectance confocal microscopy is a novel non-invasive imaging technique. It allows in vivo imaging of the skin. It allows visualization of the epidermis and superficial dermis at nearly histologic resolution.
Reflectance confocal microscopy of melanoacanthoma was initially described by Shahriari et al. in 2016 of a left mandibular black papule. A well-circumscribed lesion whose overall architecture showed keratotic debris-filled surface depressions and ridges was observed at the level of the stratum corneum of the epidermis; this correlated with the verrucous surface of the lesion. Numerous tangled bright dendritic cells were noted in the suprabasal spinous and granular layers of the hyperplastic epidermis; this feature was suggestive of that seen in melanoma with a pagetoid distribution of melanocytes throughout the epidermis. Since a melanoma was suspected based on the reflectance confocal microscopy findings, the lesion was excised.[13]
Porto et al., in 2019, also used reflectance confocal microscopy to evaluate a melanoacanthoma presenting as a 9 mm dark brown patch on the scalp. At the spinous-granular layer, a typical honeycomb pattern was present; also, there were not only epidermal projections and keratin-filled invaginations but also widespread dendritic pagetoid cells. At the dermo-epidermal junction, between the dermal papillae, there was also infiltration of dendritic cells. Melanophages were noted at the level of the papillary dermis. The presence of suprabasal dendritic cells can be observed in squamous cell carcinoma in situ, and the pagetoid spread of dendritic melanocytes can be noted in melanoma; therefore, the lesion was excised.[12]
In summary, several investigators have described the dermoscopic features and the reflectance confocal microscopic features of melanoacanthoma. Although dermoscopic findings such as a starburst pattern, a regular pigmentary network, and a cribriform pattern of ridges and fissures are typically observed, other features suggestive of melanoma are also frequently present. Similarly, features observed on reflectance confocal microscopy mimic those of melanoma. Hence, dermoscopy and reflectance confocal microscopy alone are not reliable for establishing the diagnosis of melanoacanthoma.
Treatment / Management
Sometimes confused for melanoma on visual examination, most melanoacanthomas were surgically removed either at the time of biopsy or subsequently by simple excision. Partial removal during the biopsy of a melanoacanthoma has resulted in the persistence of the preauricular lesion in one patient.[14] Also, the recurrence of a nasal melanoacanthoma was observed 6 months after it had been removed.[2]
Melanoacanthoma is benign epithelial neoplasms. Hence, other therapeutic interventional modalities that result in the removal of the epidermis should effectively treat the lesion. Therefore, cryotherapy with liquid nitrogen, particularly for smaller lesions and curettage, are potential options that might be considered.[8]
Topical 5-fluorouracil 5% cream was used to treat smaller lesions in a 58-year-old woman with multiple melanoacanthomas. She applied the medication twice daily for four weeks. However, the cream was discontinued since it caused a burning sensation at the application sites, and there was not any significant improvement of the melanoacanthomas.[9]
Differential Diagnosis
- Actinic keratosis (pigmented)
- Basal cell carcinoma (pigmented)
- Condyloma accuminata
- Lentigo maligna
- Melanocytic nevus
- Melanoma
- Pigmented spindle cell (Spitz) nevus
- Seborrheic keratosis
- Solar lentigo
- Squamous cell carcinoma (pigmented)
- Verrucous carcinoma
- Verruca vulgaris
The morphologic features of a melanoacanthoma can mimic those of other skin lesions. Melanoacanthomas most commonly resemble other pigmented cutaneous lesions such as actinic keratosis, a basal cell carcinoma, a lentigo, a melanoma, a nevus, or a seborrheic keratosis. However, microscopic evaluation of the lesion enables the diagnosis of melanoacanthoma to be established.[5][10][12]
The pathologic differential diagnosis is a pigmented seborrheic keratosis. However, in seborrheic keratosis, the pigment and melanocytes are predominantly present and restricted to the basal layer or lower layers of the epidermis. In contrast, in a melanoacanthoma, the pigment results from the dendritic melanocytes that are present throughout not only the lower layers but also the upper layers of the epidermis. The absence of cellular atypia and numerous mitoses excludes squamous cell carcinoma. The absence of koilocytes excludes the diagnosis of verrucae.
Actinic keratoses are premalignant skin lesions. They typically present as scaly red plaques on sun-exposed areas. However, pigmented actinic keratoses, usually in patients with darker skin color, can present as a flat dark brown or black hyperpigmented plaque that mimics other pigmented lesions such as melanoma or seborrheic keratosis. Although the diagnosis may be suspected clinically, it is often established after the lesion has been biopsied. Management is similar to non-pigmented actinic keratoses, including destructive modalities (such as liquid nitrogen cryotherapy or curettage or superficial chemical peels), topical treatments (with either 5-fluorouracil, imiquimod or ingenol mebutate) or photodynamic therapy.
Basal cell carcinoma is the most common skin cancer. It typically occurs on sun-exposed sites; however, the tumor can occur on areas that do not regularly get exposed to the sun, such as the areola of the breast, the axilla, the perianal region, and the umbilicus. The morphology of the cancer is variable. Superficial basal cell carcinomas present as flat red scaly plaques. Nodular basal cell carcinomas appear as flesh-colored to translucent papules or nodules with overlying and/or adjacent telangiectasias; some of the lesions may ulcerate. Sclerosing basal cell carcinomas mimic scars. However, an uncommon variant of basal cell carcinoma is pigmented and presents as a black or brown papule, plaque or nodule and may masquerade as either a melanoma. Microscopically, the pigment can be found in the basaloid tumor cells in macrophages in the dermis or both locations. Treatment of a pigmented basal cell carcinoma is similar to that of the non-pigmented tumor—often, the tumor is removed by either Mohs microscopically controlled surgery or simple excision.
Lentigo microscopically presents as hyperpigmentation of cells (including both the keratinocytes and melanocytes) in the basal layer of the epidermis; also, there may be a slight increase in the number of melanocytes. When this occurs in a sun-exposed location, the lesion is referred to as a solar lentigo; at other sites, the lesion is denoted as a lentigo simplex or just a lentigo. Clinically, the benign pigmented lesion is flat.
Melanoma is a malignant melanocytic neoplasm. The lesion may be flat (such as a lentigo maligna melanoma, which is typically found on sun-exposed sites such as the face and superficial spreading malignant melanoma) or a raised nodule (such as a nodular melanoma). Less frequently, melanomas are amelanotic and present as non-pigmented flesh-colored nodules. However, more commonly, melanomas are darkly pigmented; indeed, the individual tumors can be multicolored with black, brown, red, and even blue areas. Melanomas may spontaneously bleed or ulcerate or both.
A melanocytic nevus is a benign lesion. Common subtypes include junctional, intradermal, and compound. Whereas the intradermal nevus is flesh-colored and only contains nests of melanocytes in the dermis, the junctional nevus (which is a flat lesion with melanocyte nests restricted to the dermo-epidermal junction) and compound nevus (which is a raised lesion with melanocyte nests not only found along the dermo-epidermal junction but also in the dermis) are pigmented.
Seborrheic keratoses are benign pigmented plaques that are usually acquired in adulthood. Although an individual may have only one or a few lesions, many patients have numerous lesions. Their morphologic appearance is either flat (macular) or raised (plaque) with a ‘stuck on’ appearance. Rarely, they may be the cutaneous harbinger of an occult visceral malignancy when the new onset of multiple lesions suddenly appears; this has been referred to as the ‘sign of Leser-Trelat.’ Microscopically, seborrheic keratoses can share similar to melanoacanthoma; however, seborrheic keratoses only consist of keratinocytes, whereas melanoacanthomas are comprised of both keratinocytes and melanocytes throughout the entire lesion.
Squamous cell carcinoma and squamous cell carcinoma in situ both typically present as red plaques or nodules. In contrast, pigmented squamous cell carcinoma and pigmented squamous cell carcinoma in situ present as brown tumors. They are often located in sun-exposed locations of the body. However, the pigmented lesions are often mistaken to be either benign or malignant melanocytic lesions.
Prognosis
The prognosis for an individual with a melanoacanthoma is excellent. The skin lesion is non-cancerous. There are no systemic manifestations associated with melanoacanthoma.
Most patients have had their melanoacanthoma removed either during the biopsy or after the diagnosis has been established. Several of these individuals have had clinical follow-up after their melanoacanthoma has been excision. There was no recurrence of the benign neoplasm in nearly all of these individuals.[11][14][16]
However, the recurrence or persistence of melanoacanthoma has been described in three individuals. A 54-year-old White woman presented with a 13 by 16 millimeter, black lesion on the bridge of her nose of 18 months duration. The clinical diagnosis of a seborrheic keratosis was considered, and the lesion was removed. Within 6 months after the removal of the melanoacanthoma, the lesion reappeared.[2]
A 65-year-old African-American woman presented with a 2 by 1 centimeter, black plaque on her right preauricular area. An intradermal nevus was suspected clinically, and a superficial shave biopsy was performed. Microscopic examination showed a melanoacanthoma that extended to the deep margin of the specimen. The woman declined further treatment; yet, at follow up several months later, the melanoacanthoma had not only persisted but also increased in size. The residual lesion was completely removed after a deep shave biopsy. There has been no subsequent recurrence after the biopsy site healed.[14]
Complications
The melanoacanthoma is a benign epithelial lesion. To date, there has not been any observation of malignant transformation. Similarly, in contrast to seborrheic keratoses, which can occur adjacent to or above a skin cancer, there has been no report of a cutaneous collision tumor consisting of a melanoacanthoma and a malignant skin neoplasm.
Although most melanoacanthomas grow slowly, they can become giant, measuring over 10 cm in greatest diameter. Also, albeit less common, some melanoacanthoma has been noted to grow rapidly. In either of these settings, the lesion can become irritated and tender from rubbing against the adjacent skin or the overlying clothing.
Rarely, a larger melanoacanthoma may spontaneously ulcerate. In one woman, the melanoacanthoma on her back ulcerated and thick yellow-colored pus drained from the lesion. She had difficulty lying down, and the situation did not resolve after multiple courses of antibiotics. Subsequently, excision and skin grafting was planned; however, she declined the surgery.[9]
Deterrence and Patient Education
A melanoacanthoma is a benign skin lesion. However, its clinical morphology mimics not only other benign epithelial tumors such as a seborrheic keratosis but also malignant neoplasms such as melanoma. Therefore, it is essential that older individuals, particularly those 60 years of age and older -who either discover a new black skin lesion or witness a pigmented cutaneous lesion that may have been present for several years but have continued to enlarge to seek additional evaluation.
Non-invasive methods of examination--including clinical inspection, dermoscopy, and reflectance confocal microscopy—can be performed. However, it is difficult to establish the diagnosis of melanoacanthoma using these modalities since features of melanoma can be observed with each of these methods of investigation. Therefore, when the possibility of a melanoacanthoma is considered, an appropriate biopsy of the lesion should be performed to confirm the diagnosis and to exclude other conditions.
Pearls and Other Issues
Melanoacanthoma is a rare benign lesion of the epidermis; however, its actual incidence remains to be established. Melanoacanthoma is seldom the diagnosis considered by the clinician who evaluates the patient; most often, when a biopsy specimen is submitted, either melanoma or a seborrheic keratosis or both was the diagnosis suggested. Complete removal of the melanoacanthoma is recommended since the lesion may persist following partial removal.
Enhancing Healthcare Team Outcomes
The clinical morphology of a melanoacanthoma can mimic that of another benign skin tumor, a seborrheic keratosis. However, for many of the patients in whom this epithelial neoplasm has been described, the initial impression was a melanoma. Therefore, the patient’s primary health care professional, either physician (such as a family practice doctor or internist) or nurse practitioner, should consider performing a complete skin check of the patient, especially those over 60 years of age.
The detection of a new or progressively enlarging asymptomatic black lesion should prompt the primary health care professional to refer the patient to a dermatologist or surgeon for evaluation, including biopsy, of the lesion.
Appropriate postoperative care, after the biopsy has been done, should be initiated to promote healing of the wound without infection. This management may involve either the personnel in the office where the biopsy was performed or visiting nurses or both.
Periodic follow up by the clinician to confirm healing and ensure that there is no persistence or recurrence of the melanoacanthoma. [Level 5]
(Click Image to Enlarge)

Figure 4. Melanoacanthoma on the left lower abdomen of an 85-year-old man. Distant (A) and closer (B) views of a melanoacanthoma (black arrows) presenting as a large 3 x 2.5 cm lobulated, exophytic, black nodule with hyperpigmentation of the surrounding skin on the left lower abdomen.
The figure and legend were originally published in Cureus under the Creative Commons (CC-BY): https://creative commons.org/licenses/ on June 25, 2019 (Guiterrez N, Erickson CP, Calame A, Sateesh BR, Cohen PR: Melanoacanthoma masquerading as melanoma: case reports and literature review. Cureus. 2019 Jun 25:11(6):e4998) and are republished with permission.
(Click Image to Enlarge)
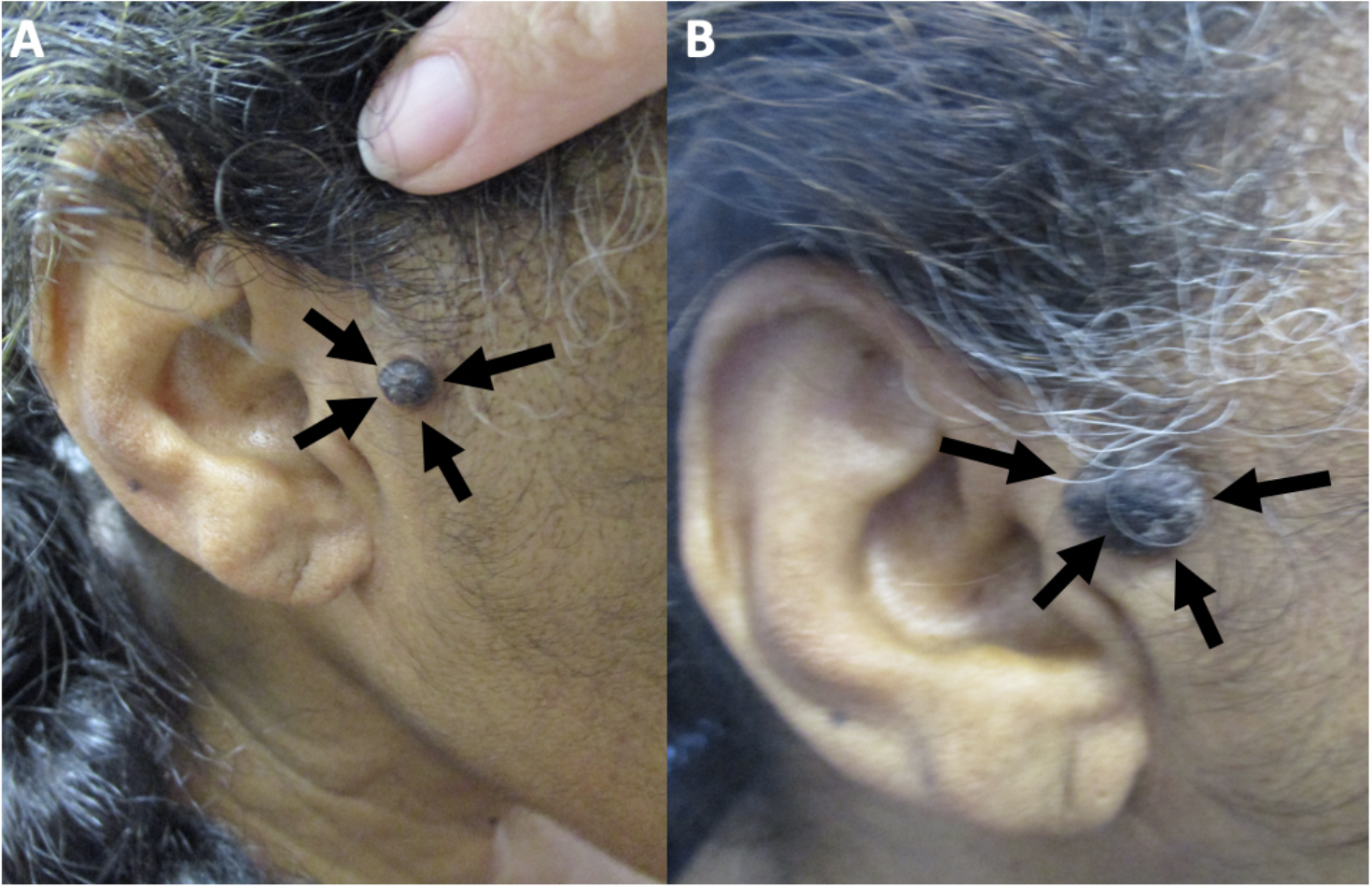
Figure 1. Clinical presentation of melanoacanthoma on the right preauricular area of a 65-year- old woman. Clinical presentation of the initial (A) and recurrent (B) melanoacanthoma (black arrows) on the right preauricular area. The tumor initially presented as a 2 x 1 cm black plaque (A). The lesion not only persisted but also increased in size, morphologically mimicking a melanoma (B).
The figure and legend were originally published in Cureus under the Creative Commons (CC-BY): https://creative commons.org/licenses/ on June 25, 2019 (Guiterrez N, Erickson CP, Calame A, Sateesh BR, Cohen PR: Melanoacanthoma masquerading as melanoma: case reports and literature review. Cureus. 2019 Jun 25:11(6):e4998) and are republished with permission.
(Click Image to Enlarge)
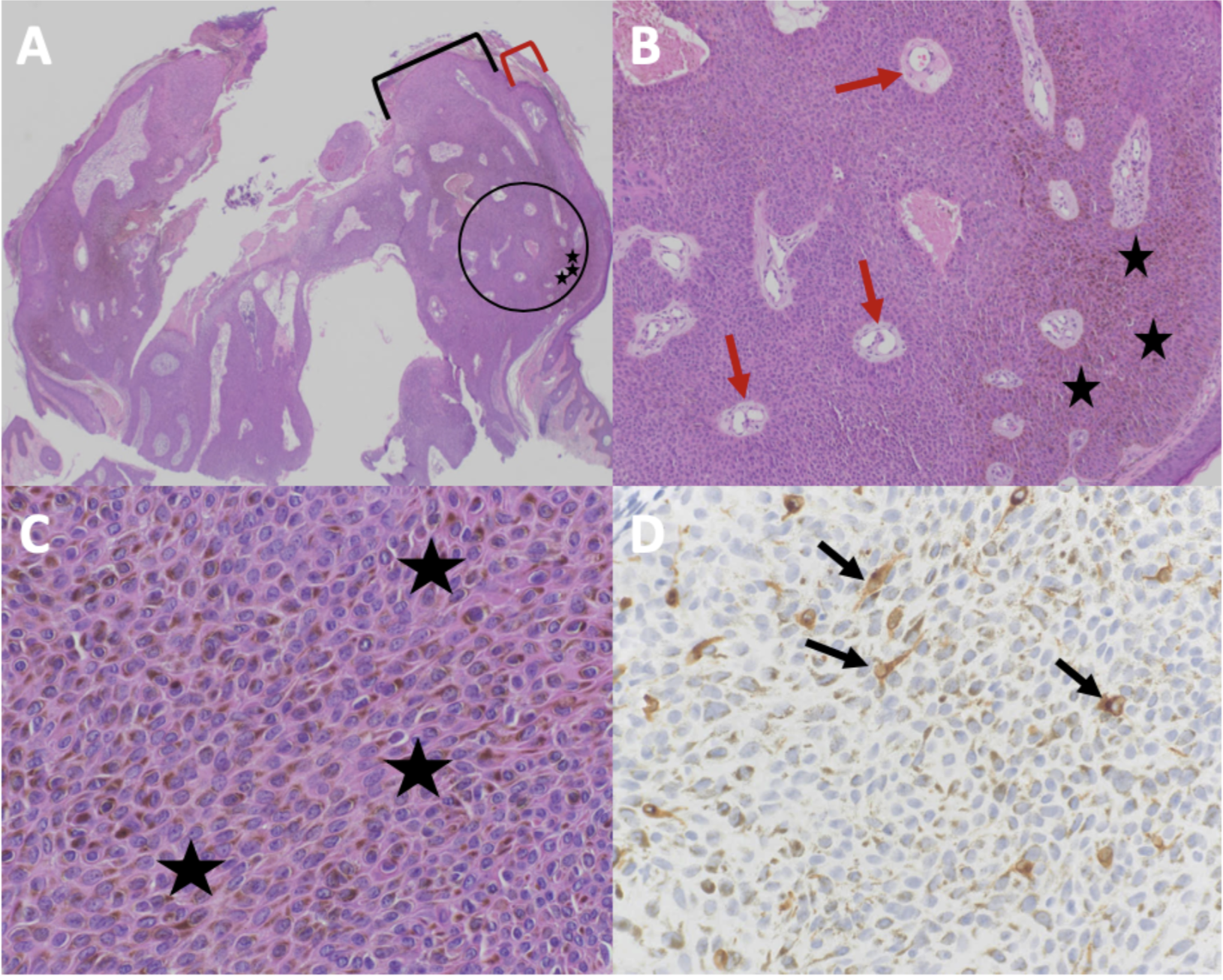
Figure 2. Microscopic presentation of melanoacanthoma on the right preauricular area. Low (A) and higher (B and C) magnification of hematoxylin and eosin (H&E) stained sections shows an exophytic nodule with hyperkeratosis (thickening of the stratum corneum as shown between the red bracket) and acanthosis (thickening of the epidermis as shown between the black bracket); the area enclosed in the black circle of image A is shown at higher magnification in image B. There is hyperpigmentation throughout all layers of the epidermis (black stars). Tangential sectioning of the tumor shows small areas of dermis, containing epithelial lined vessels and erythrocytes within the epithelium (red arrows). There is lymphocytic perivascular inflammation in the dermis. A higher magnification view (D) of MART-1 stained section shows positive staining of dendritic melanocytes throughout all layers of the epidermis (black arrows) (H&E: A, x2; B, x10; C, x40; MART-1 immunoperoxidase; D, x40).
The figure and legend were originally published in Cureus under the Creative Commons (CC-BY): https://creative commons.org/licenses/ on June 25, 2019 (Guiterrez N, Erickson CP, Calame A, Sateesh BR, Cohen PR: Melanoacanthoma masquerading as melanoma: case reports and literature review. Cureus. 2019 Jun 25:11(6):e4998) and are republished with permission.
(Click Image to Enlarge)
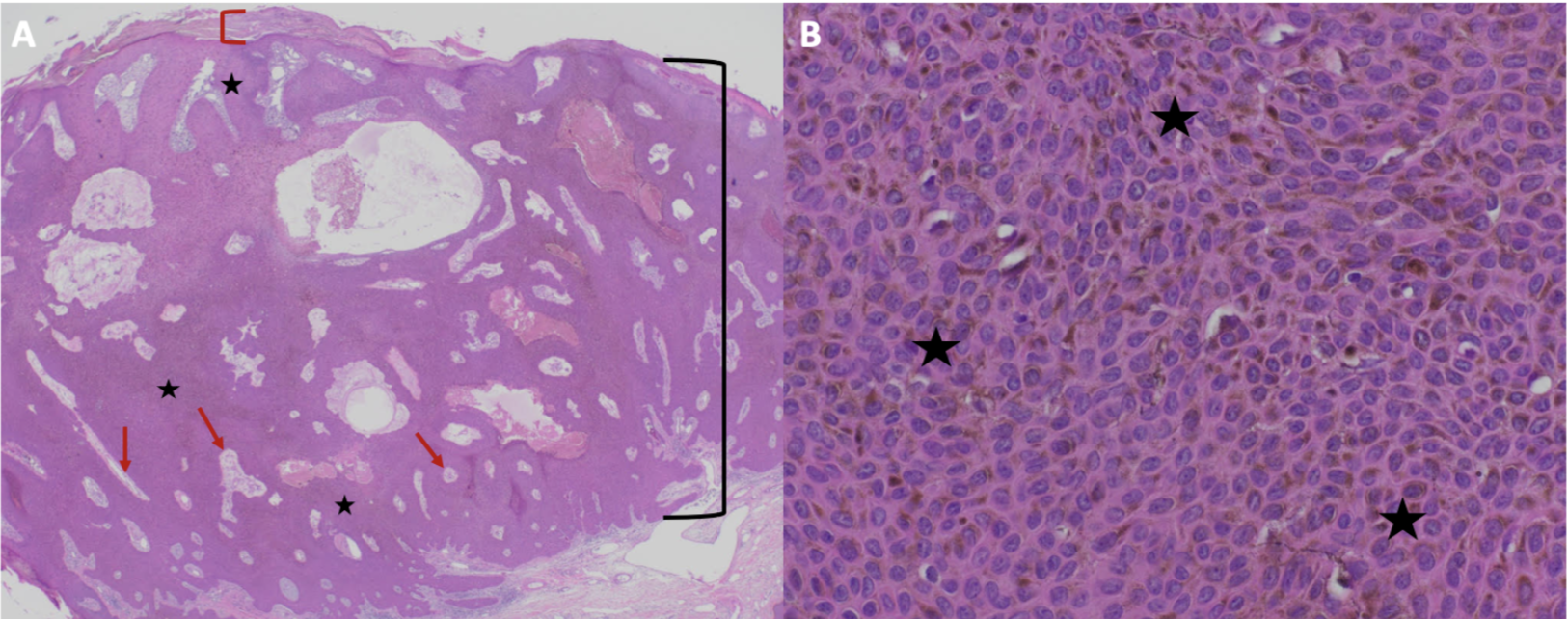
Figure 3. Microscopic presentation of recurrent melanoacanthoma on the right preauricular area of a 65-year-old woman. Distant (A) and closer (B) views of pathologic features of a melanoacanthoma. Low (A) and higher (B) magnification of hematoxylin and eosin stained sections show an exophytic nodule with hyperkeratosis (thickening of the stratum corneum as shown between the red bracket) and acanthosis (thickening of the epidermis as shown between the black bracket). There is hyperpigmentation throughout all layers of the epidermis (black stars). Tangential sectioning of the tumor shows small areas of dermis, containing epithelial lined vessels and erythrocytes within the epithelium (red arrows). There is lymphocytic perivascular inflammation in the dermis. (Hematoxylin and eosin: A, x2; B, x40).
The figure and legend were originally published in Cureus under the Creative Commons (CC-BY): https://creative commons.org/licenses/ on June 25, 2019 (Guiterrez N, Erickson CP, Calame A, Sateesh BR, Cohen PR: Melanoacanthoma masquerading as melanoma: case reports and literature review. Cureus. 2019 Jun 25:11(6):e4998) and are republished with permission.
(Click Image to Enlarge)
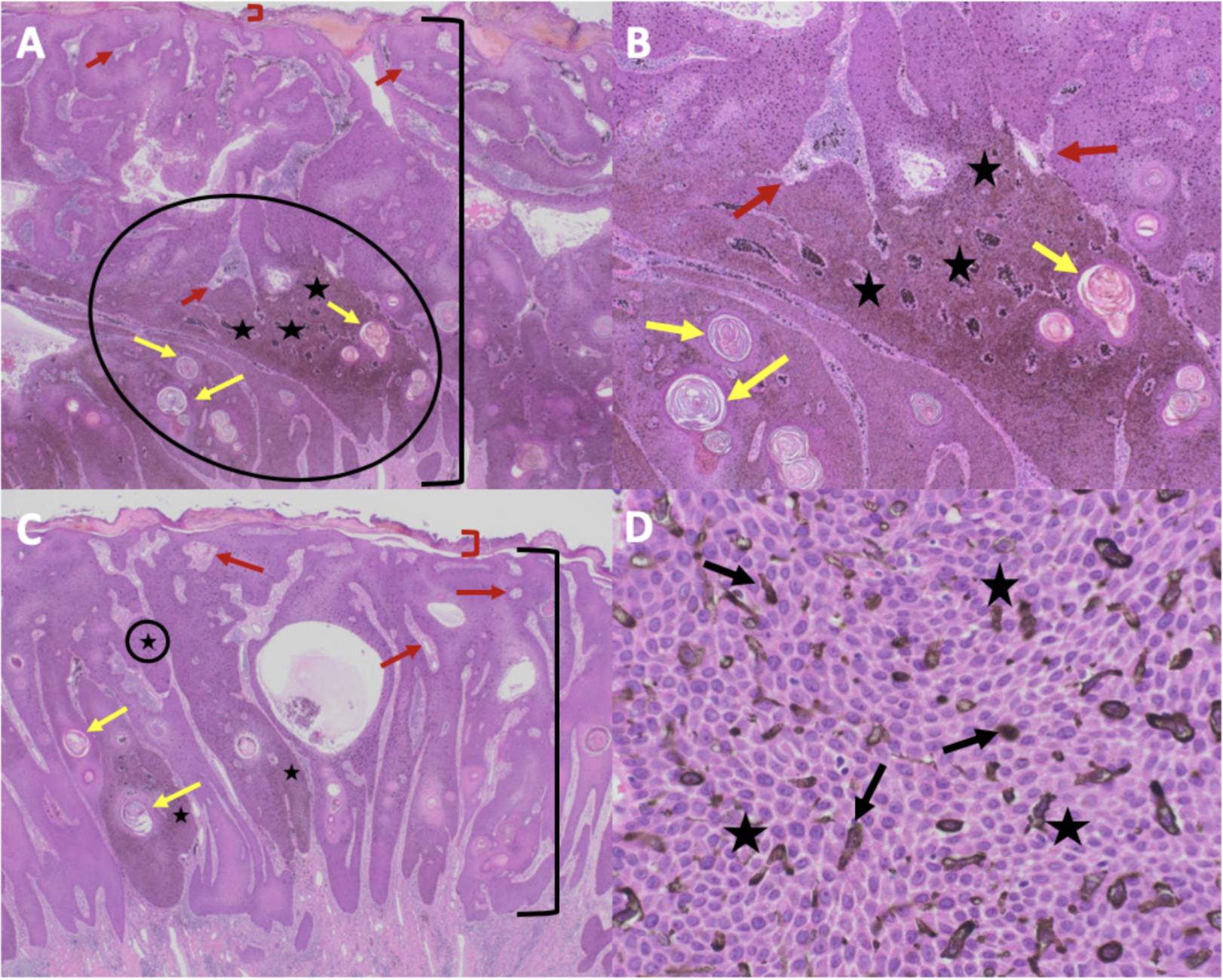
Figure 5. Microscopic presentation of melanoacanthoma on the left lower abdomen os an 85-year-old man. The melanoacanthoma contains exophytic (A and B) and endophytic (C and D) portions; the circled areas in A and C are shown in B and D, respectively. There is hyperkeratosis (between the red bracket) and acanthosis (between the black bracket). There is hyperpigmentation throughout all layers of the epidermis (black stars). Tangential sectioning of the tumor shows not only keratin-filled pseudocysts (yellow arrows) but also small areas of dermis containing epithelial lined vessels and erythrocytes within the epithelium (red arrows). There is lymphocytic perivascular inflammation in the dermis. The higher magnification view (D) also shows dendritic melanocytes throughout all layers of the epidermis (black arrows) and hyperpigmentation throughout all layers of the epidermis (black stars) (Hematoxylin and eosin: A, x2; B, x10; C, x40; D, x40).
The figure and legend were originally published in Cureus under the Creative Commons (CC-BY): https://creative commons.org/licenses/ on June 25, 2019 (Guiterrez N, Erickson CP, Calame A, Sateesh BR, Cohen PR: Melanoacanthoma masquerading as melanoma: case reports and literature review. Cureus. 2019 Jun 25:11(6):e4998) and are republished with permission.