Miliary Tuberculosis
- Article Author:
- Shekhar Vohra
- Article Editor:
- Harpal Dhaliwal
- Updated:
- 8/25/2020 6:39:52 PM
- For CME on this topic:
- Miliary Tuberculosis CME
- PubMed Link:
- Miliary Tuberculosis
Introduction
Miliary tuberculosis is a potentially fatal form of the disseminated disease due to the hematogenous spread of tubercle bacilli to the lungs, and other organs. It results in the formation of millet seed-sized (1 to 2 mm) tuberculous foci. The term miliary tuberculosis was first coined by John Jacobus Manget in 1700 while describing a pathological specimen having tiny tubercles similar to millet seeds in appearance. It originated from Latin word miliarius, related to millet seed. Disseminated tuberculosis is defined as simultaneous involvement of at least two non-contiguous organ sites of the body, or infection of the blood, bone marrow or liver. Miliary mottling on a chest radiograph is the classical hallmark which supports the diagnosis of miliary tuberculosis. Miliary tuberculosis is classified as both pulmonary and extrapulmonary tuberculosis.[1][2]
Etiology
Miliary tuberculosis is caused by Mycobacterium tuberculosis. The human host serves as a natural reservoir. M. bovis, M. africanum, and M. microti, together with M. tuberculosis, are known as the Mycobacterium tuberculosis complex. Other mycobacteria organisms are classified as non-tuberculous or atypical mycobacterial organisms. M. tuberculosis is a non-spore forming, non-motile, obligate-aerobic, facultative, catalase-negative, intracellular bacteria. It is gram stain neutral and stained by Ziehl-Neelsen stain. The presence of mycolic acids in the cell wall imparts them acid fastness when decolorized with acid-alcohol and are thus known as alcohol and acid-fast bacillus (AAFB).[3]
Epidemiology
The epidemiological patterns of military tuberculosis have been altered due to the widespread use of immunosuppressants, biological agents and chemotherapy, in addition to the increasing prevalence of diabetes mellitus, chronic kidney disease, alcoholism, organ transplantation, the emergence of HIV/AIDS pandemic, and immigration from high endemic countries. In 2018, the World Health Organization Global Tuberculosis report estimated that ten million people developed TB, and 1.3 million died from the disease globally.[4]
According to the Center for Disease Control and Prevention, 8920, new tuberculosis cases were reported in the United States for the year 2019.[5] Miliary tuberculosis accounts for about 1% to 2% of all cases of tuberculosis and up to 20% of all forms of extrapulmonary tuberculosis in immunocompetent individuals. Before the advent of antibiotics, miliary tuberculosis was thought to be a disease of infants and children. However, currently, the disease has bimodal age distribution, with one peak occurring in young adults and adolescents and the second peak occurring in the elderly with slight male preponderance.[6]
Histopathology
When examined grossly, miliary tuberculosis is characterized by small, punctate, gray to reddish-brown, rounded lesions of the more or less uniform size seen in the lungs, and various other organs. In an immunocompetent individual, microscopically, each focus represents a typical tubercle with caseation necrosis in the center having tubercle bacilli surrounded by typical Langhans type giant cells, epithelioid cells, lymphocytes, and fibrocytes. However, in immune-suppressed individuals, the foci show caseation with tubercle bacilli without granuloma formation, known as non-reactive miliary TB.[6][7][8]
History and Physical
The clinical features of miliary TB are usually nonspecific, protean, and remain obscure until late in the disease course. They are typically sub-acute or chronic and very rarely can present acutely. The patient's symptoms usually range from nonspecific constitutional symptoms in the form of fever, generalized weakness, weight loss, lassitude, and cough to organ-specific dysfunction secondary to tubercular involvement. The most common extrapulmonary sites include the lymphatic system, bones and joints, liver, central nervous system (CNS), and adrenal glands. Physical findings include hepatomegaly, splenomegaly, and lymphadenopathy.[6]
Clinically, two main variants of miliary TB are reported depending on the demographic profile along with clinical features, viz: classical acute miliary TB and cryptic miliary TB.
Acute Miliary TB
Acute miliary TB is seen under 40 years of age. Most of the patients present with constitutional symptoms or can have signs and symptoms related to one organ owing to the systemic nature of the disease. Evening rise of temperature and night sweats of 1-2 weeks duration are classically described, although a patient can have early morning fever spikes. Dry cough with scanty sputum and dyspnea are other common symptoms, and sometimes patients can have hemoptysis. ARDS is a rare fatal presentation of military TB.[6][9][10]
Choroid tubercles, if seen on fundus examination, provide a valuable clue to the diagnosis. Choroid tubercles are bilateral, pale, gray-white, or yellowish lesions usually less than one-quarter of the size of the optic disc and are located within 2 cm of the optic nerve. When present, choroidal tubercles are considered pathognomonic of miliary TB. They occur more commonly in children.[6][11]
Skin involvement may occur in the form of erythematous macules and papules (tuberculosis miliaria cutis).[1]
Neurological involvement occurs in the form of headache secondary to tubercular meningitis (TBM) with or without tuberculoma formation and motor or sensory abnormality because of thoracic transverse myelopathy.[6][12] TBM has been described in 10% to 30% of adult patients with miliary TB.[13]
Musculoskeletal involvement accounts for 10% of cases of extrapulmonary TB. Its most common site is vertebral involvement in the form of tubercular spondylitis (Pott's spine), followed by septic arthritis. It can also occur in the form of osteomyelitis, tenosynovitis, bursitis, and pyomyositis. Pott's spine presents clinically as constitutional symptoms, back pain, tenderness, paraplegia or paraparesis, and kyphotic or scoliotic deformities.[14]
Abdominal TB occurs following hematogenous spread from pulmonary focus or by local spread from the gastrointestinal tract or abdomen as the primary source. Abdominal TB can occur as hepatic, intestinal, or peritoneal involvement.[15] Hepatic involvement presents as abdominal pain localizing to the right upper quadrant, nausea, vomiting, fever, and generalized fatigue. Icterus and hepatosplenomegaly are seen clinically.[16][17] Intestinal TB usually presents with fever, failure to thrive, micro and macronutrient deficiencies, altered bowel habits, subacute to acute intestinal obstruction. TB peritonitis should be suspected in a patient presenting with complaints of fever, fatigue, abdominal pain, and ascites.[15]
Miliary TB can present as overt adrenal insufficiency (Addison's disease) at the time of initial presentation, or during anti-tubercular treatment.[6][18]
Clinically significant cardiac and renal involvement is uncommon; however, it can cause myocarditis, congestive heart failure, endocarditis, mycotic aneurysms, and acute kidney injury either directly or as a syndromic constellation of multiorgan dysfunction syndrome (MODS).[6][19]
Even though the clinical presentation of miliary TB in children is similar to that of adults, but they do have important differences. Miliary TB develops less frequently in children who have received the BCG vaccination. In children with miliary TB, chills, night sweats, hemoptysis, and productive cough have been reported less frequently, while peripheral lymphadenopathy and hepatosplenomegaly are more common compared to adults. TBM is more frequently seen in children with miliary TB (20%–40%) compared to adults (15% to 30%).[6][20]
The clinical presentation of TB in HIV patients depends on their CD4+ count. Patients having CD4+ count >200 cells/mm have their disease progression similar to immunocompetent individuals. When the CD4+ count is < 200 cells/mm or profound immunosuppression due to any cause, atypical manifestations of miliary TB occur in the form of cutaneous involvement, intrathoracic lymphadenopathy, and tuberculin anergy.[6][21]
Cryptic Miliary TB
It occurs primarily in the elderly over 60 years of age. The clinical presentation involves the absence of fever, progressive weight loss, and general debility as the dominant clinical feature mimicking a metastatic carcinoma, along with mild hepatosplenomegaly. The patients have a normal chest radiograph and negative skin tuberculin test, causing a delay in diagnosis.[6][22][23]
Evaluation
Laboratory Findings
Hematological changes seen in miliary TB are usually nonspecific. The patient can have pancytopenia, anemia, leucopenia, leucocytosis predominantly lymphocytosis, thrombocytopenia, or thrombocytosis. The most common hematological abnormality encountered in miliary TB is anemia of chronic disease. Elevated acute phase reactants in the form of erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are frequently encountered. Leukaemoid reaction is also described and can be mistaken for leukemia. Disseminated intravascular coagulation (DIC) is rare and seen in the setting of MODS and ARDS.[6][24][25][26]
Biochemistry panels can be normal or have subtle disturbances. Hyponatremia, secondary to meningeal involvement or abnormal antidiuretic hormone levels, is frequently encountered and is an indicator of neurological involvement. Hyperbilirubinemia, hypoalbuminemia (negative acute phase reactant), and elevated alkaline phosphatase are also seen. Hypercalcemia, though rare, has also been reported.[6][24][27][28][24]
Diagnosis
Due to the high morbidity and mortality associated with the disease, it is pertinent for the clinician to have a high index of suspicion for the disease even in the setting of varied clinical presentations. A multi-pronged approach comprising of meticulous history taking, thorough clinical examination, radiological, and laboratory investigation is required for the early diagnosis and adequate treatment.
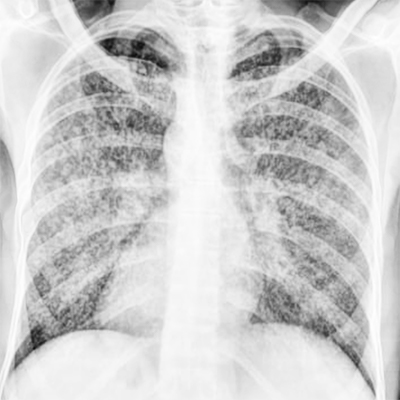
There are no uniform guidelines for the diagnosis of miliary tuberculosis. The following criteria have been suggested for the diagnosis of miliary TB: (i) clinical presentation consistent with a diagnosis of TB, such as pyrexia with evening rise of temperature, weight loss, anorexia, tachycardia, and night sweats of greater than six weeks duration responding to the antituberculosis treatment; (ii) classical miliary pattern on chest radiograph; (iii) bilateral diffuse reticulonodular lung lesions on a background of miliary shadows demonstrable either on plain chest radiograph or HRCT; and (iv) microbiological, cytopathological, histopathological, or molecular evidence of TB.[6]
The chest radiograph shows typical miliary mottling, i.e., homogenously distributed discrete uniform size (1 to 2 mm) millet-shaped lesions in all lung zones, which is also the hallmark for making the diagnosis of miliary TB.[1][6] However, in the early stages of the disease or cryptic TB, the same cannot be seen, for which high-resolution chest tomography (HRCT) is advisable for parenchymal lesions, whereas contrast-enhanced CT (CECT) detects lymphadenopathy, calcification, and pleural lesions better.[6][24][29][30][29] In extrapulmonary locations, ultra-sonography, CECT, and MRI are useful in diagnosing the extent of organ involvement.[31] Recently, positron-emission tomographic CT has been an investigating tool for the evaluation of suspected TB.[32][33][34]
Tuberculin skin testing (TST) shows anergy in miliary TB more frequently than pulmonary TB, but it can become positive during antituberculosis therapy. A positive interferon-gamma release assay (IGRA) indicates the only infection and does not signify active disease; thus, its use is limited in highly endemic areas.[35] On fundoscopy, the presence of choroid tubercles is a diagnostic of miliary TB.[24]
Invasive diagnostic procedures are indicated for patients with suspected extrapulmonary TB. In addition to testing of specimens from the involved sites (e.g., CSF for tuberculous meningitis, pleural fluid, ascitic fluid, gastric aspirate, urine and pus from a cold abscess), biopsy and culture of bone marrow and liver tissue have a good diagnostic yield in miliary TB. Image-guided radiological procedures, such as fine-needle aspiration for cytological examination (FNAC) and biopsy under CT or MRI guidance, are useful for procuring tissue and body fluids for diagnostic testing.[6][24][36][37]
The definitive diagnosis of TB depends on the isolation and detection of mycobacterial isolates from a clinical specimen (sputum, body fluids, tissue, and biopsy samples) and by inoculating it on agar-based (e.g., Lowenstein-Jensen) or liquid media having fluorescence detection. Samples are evaluated for direct visualization of acid-fast bacilli using ZN staining or auramine-rhodamine (fluorochrome dye) staining, which is more sensitive than conventional ZN staining.[38][39][38] The specimen should be cultured on standard solid LJ media along with inoculation in liquid media (e.g., BACTEC MGIT 960).[19][40] The advantage of using liquid-based media has reduced the time for diagnosis from 6-8 weeks on solid media to 1-3 weeks on liquid media along with simultaneous drug sensitivity testing for drug resistance. Blood culture examination is not employed for mycobacterium isolation. They are usually negative, although positive results can be observed in immunocompromised individuals with hematogenous dissemination.[41] M. tuberculosis can be differentiated from isolated non-tuberculous mycobacteria by hybridization using nucleic acid probes or biochemical methods or by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF).[42]
Histopathological examination of a tissue biopsy specimen is characteristic and shows granulomatous inflammation with central caseation, with or without tubercle bacilli. Demonstration of tubercle granuloma in the biopsy sample in a typical clinical scenario is suggestive of tuberculosis; however, either demonstration of bacilli by staining or by culture is required to confirm the diagnosis.[6][43]
Application of molecular methods such as PCR, Gene Xpert MTB/RIF, and line probe assays (LPA) can be useful for the early diagnosis of pulmonary and extrapulmonary TB along with the detection of drug resistance in tubercle bacilli. These techniques are advantageous in a way that test results may be available within hours and can be used on a wide array of specimens for the diagnosis of TB and drug resistance.[43][44][45][46][47]
Serological tests are not advocated for the detection of TB because of low sensitivity and specificity. However, adenosine deaminase (ADA) and interferon-gamma (IFN- γ) can be used as an adjunct for the diagnosis of tuberculosis in pleural, pericardial, and ascitic fluids.[24][48][49][50][51] ADA has also found utility in the diagnosis of tubercular meningitis.[52]
The cut-off value of ADA for pleural effusion and pericardial effusion is 40 U/L, for tubercular ascites 39 U/L, and for CSF is 10 U/L.[53][54][48][55] ADA levels could be falsely elevated in cases of empyema, parapneumonic effusions, malignancies such as lymphoma, endometriosis, and collagen vascular disorder.[53] High CSF ADA can also be seen in cerebral malaria, brucellosis, neurosarcoidosis, fulminant pyogenic meningitis, AIDS, and CSF lymphoma.[56]
Treatment / Management
The use of a standard antitubercular drug regimen of pulmonary TB is also applicable to the treatment of miliary tuberculosis. As per the World Health Organisation, a standard 6-month treatment regimen consists of two months of intensive phase treatment with isoniazid, rifampicin, pyrazinamide and ethambutol, and four months of continuation phase with isoniazid and rifampicin.[24]
The duration of treatment can be modified according to the age group affected and the site of the primary disease. Longer duration of treatment is advisable for children, immunocompromised individuals, patients with slow clinical response, tubercular meningitis, tubercular lymphadenitis, and skeletal TB. The generally recommended minimum duration of therapy is nine months for skeletal TB, and 12 months for TBM.[57][58][59] For abdominal TB, nine months of therapy used to be the conventional approach. However, a recent multicenter randomized trial showed that both 6-months and 9-months of therapy are equivalent.[60]
It is important to suspect and document the neurological involvement (particularly TBM) in all cases of miliary TB for two reasons: To ensure the adequate duration of therapy (which is generally 12 months in TBM) and the need for concomitant steroid therapy.[61]
For previously treated patients, the WHO guidelines advocate that specimens for culture and drug susceptibility testing (DST) be obtained from all previously treated TB patients at or before the start of treatment. DST should be performed for at least isoniazid and rifampicin, and in settings where rapid molecular DSTs are available, the DST results should guide the choice of regimen. The duration of treatment can also be individualized according to the clinical setting encountered.[24]
It is advisable to screen for diabetes and HIV status of the patient suspected of miliary TB before the administration of antitubercular treatment and vice-versa. Anti-retroviral treatment (ART) for all HIV-positive TB patients within the first eight weeks of starting TB treatment and within two weeks in profoundly immunosuppressed HIV-positive TB patients with low CD4 counts less than 50 for fear of immune reconstitution inflammatory response (IRIS).[24][62][63]
Necessary medical and surgical interventions are advisable both for diagnostic and therapeutic purposes. Mechanical ventilation for the complication of ARDS, GI surgery for small bowel perforation, and ventriculoperitoneal shunt surgery for TBM can be done to decrease the complications of TB.[6]
Although there is a lack of substantial evidence, the use of corticosteroids in miliary TB has shown clinical efficacy in a few scenarios, namely adrenal insufficiency, TB meningitis, large pericardial or pleural effusion, immune reconstitution inflammatory syndrome (IRIS), Acute respiratory distress syndrome (ARDS), immune-complex nephritis and secondary hemophagocytic syndrome. [6][64][65]
Liver function tests (LFTs) should be done both at the start of ATT and during the course of treatment. Serial LFTs should be done to check for ATT induced hepatitis. The criteria for ATT induced hepatitis has evolved over time. It is considered when, in the absence of symptoms, there is an elevation of transaminases up to 5 times the upper limit of normal (ULN) and in the presence of symptoms up to 3 times the ULN or bilirubin rises twice the ULN, provided competing causes such as acute viral hepatitis, autoimmune hepatitis, and others liver diseases are ruled out.
Once the patient is found to have ATT induced hepatitis, all hepatotoxic drugs (isoniazid, rifampicin, and pyrazinamide) should be stopped, and the patient is started on modified ATT to prevent further liver damage. Rechallenge of ATT can be done according to either British Thoracic Society guidelines, or American Thoracic Society guidelines once the LFTs have normalized. They advocate either for an incremental increase of ATT drugs or re-introduction at full dosage.[66][67]
Differential Diagnosis
Varied clinical etiologies can present as a miliary pattern on chest radiography and CT, and therefore thorough workup is required for reaching an etiological conclusion. Apart from miliary tuberculosis, other causes of miliary shadowing include histoplasmosis, blastomycosis, coccidioidomycosis, nocardiosis, sarcoidosis, carcinoma lung with lymphangitis carcinomatosis, metastatic carcinoma, the spread of pyogenic infection from a remote site, pulmonary hemosiderosis and hypersensitivity pneumonitis.[6][24][68]
Prognosis
Miliary tuberculosis has high morbidity and mortality if not managed properly. Delay in diagnosis and lack of initiation of specific antituberculosis treatment appears to be the most important factor responsible for mortality. The mortality related to miliary TB is about 15 to 20 % in children and is slightly higher in adults [25% to 30%].[20][69][70][71]In patients with ARDS due to miliary TB, an Acute Physiology and Chronic Health Evaluation (APACHE II) score greater than 18 or a score less than or equal to 18 in the presence of hyponatremia and ratio of arterial oxygen tension (PaO2) to the fraction of inspired oxygen (FIO2) less than or equal to 108.5 have been identified to be the predictors of death.[72]
Complications
Because of the extensive clinical spectrum of tuberculosis compounded by the HIV burden along with the use of immunosuppressive drugs, any delay in the diagnosis can wreak havoc for the patient. Delayed administration of the treatment can lead to various complications, namely
- ARDS
- Multi-organ dysfunction syndrome (MODS)
- Tubercular empyema
- Air leak syndromes (pneumothorax and pneumomediastinum)
- Tubercular pericardial effusion and pericarditis
- Immune reconstitution inflammatory syndrome (IRIS)
- Myocarditis, native and prosthetic valve endocarditis, and intracardiac masses
- Mycotic aneurysm of the aorta
- Tubercular meningitis with focal neurological deficits
- Systemic amyloidosis
- Immune complex glomerulonephritis
- Bone marrow suppression, disseminated intravascular coagulation (DIC)[24]
Deterrence and Patient Education
Patient education and counseling are the cornerstones for the management of tuberculosis. Proper education materials should be provided to the at-risk population to increase the early detection of tuberculosis. Once a patient is diagnosed with tuberculosis, importance should be given to the fact that the patient is being provided with adequate information regarding the disease they are suffering from and the ways they could adopt to prevent the transmission of the disease to their families. The patient must be taught about the proper drug regimen, the importance of treatment compliance, the symptoms of any drug toxicities, and to communicate it to the healthcare provider for the proper optimization of the drug regimen.
Pearls and Other Issues
The development of the BCG vaccine and its utilization in the underdeveloped countries have helped in decreasing pediatric tuberculosis. However, we still need to develop newer drugs and ways to fight the disease and its ever-growing drug resistance and associated complications. Awareness campaigns regarding the disease symptoms and its adequate treatment aimed at sensitizing the general population, along with the proper handling of patients and their samples by healthcare professionals, are of utmost importance for effective handling of the disease burden. Proper screening of latent tuberculosis is necessary for susceptible populations to decrease disease incidence. Newer strategies need to be envisaged for early detection, adequate treatment, and proper management of the complications.
Enhancing Healthcare Team Outcomes
An interprofessional team that provides a holistic and integrated approach for the early diagnosis and treatment of miliary tuberculosis can help achieve the best possible outcomes for the patient. Well-structured history taking and early recognition of symptoms by the physicians, proper handling of the patient by the nurses, and adequate precautions being taken by the laboratory personnel for the samples provided for detection of tuberculosis are a must for stopping the spread of the disease in the hospital and community.
Early administration of antitubercular therapy under guidance, also described as DOTS (Directly Observed Treatment, Short-course), is vital for the resolution of the disease, and prompt recognition of any side-effect of antitubercular therapy is essential. The pharmacist's role becomes vital in the management of antitubercular treatment-induced complications and the usage of modified doses and other drugs to continue the treatment with minimal side-effects of the therapy.
Collaboration shared decision making and communication are critical elements for a good outcome. The interprofessional care provided to the patient must use an integrated care pathway combined with an evidence-based approach to disease evaluation and management.