Ocular Melanoma
- Article Author:
- Dharti Patel
- Article Editor:
- Bhupendra Patel
- Updated:
- 5/24/2020 8:02:00 PM
- For CME on this topic:
- Ocular Melanoma CME
- PubMed Link:
- Ocular Melanoma
Introduction
Melanoma is a malignant, overproliferation of melanocytes. Ocular melanoma is the second most common type of melanoma after cutaneous and is the most common primary intraocular malignant tumor in adults.
The prognosis of uveal melanoma has remained unchanged over the past few decades. The systemic prognosis depends on the size and other characteristics of the lesion. It is not affected by the choice of local treatment. This suggests dissemination at an early stage. [1] Ocular melanoma tends to spread hematogenously, which almost always involves the liver. [2]
Etiology
Common risk factors that have been linked to ocular melanoma include light skin color, atypical cutaneous nevi, light eye color, iris nevi, and freckles. Occupational sun exposure, tanning, and outdoor activities have also been associated with ocular melanoma. [3]
There has also has been a genetic correlation with uveal and cutaneous melanoma at the CLPTM1L locus.[4] Different genetic mutations have been associated with uveal melanoma. There have been studies showing the mutations encoding for the G-protein alpha-subunit[5]
Epidemiology
There is no known cause, though incidence is highest among people with lighter skin and blue eyes. Uveal melanoma affects about 6 to 8 persons per million each year in the western world. Less than 2% of patients show evidence of metastasis at presentation; over 40% of patients will die from widespread disease. [1] The most common ocular melanoma is uveal melanoma. There has been a 30% greater incidence of ocular melanoma in females than males. There has been a higher association in non-Hispanic whites, approximately 6.02 million, with African Americans having 0.31 per million, and Asians having 0.39 per million incidences. [5]
Pathophysiology
Mechanisms of how ocular melanoma can develop are the oxidative damage done to pigmented tissues, which are controlled by the degree and type of iris pigmentation.[4] Other theories that have been associated with the development of uveal melanoma is the fundamental genetic sequencing of the pairing bases from adenine-to-cytosine mutations, whereas ciliochoroidal melanoma has been associated with adenine-to-thymine mutations.[5]
Melanomas may also arise from de-novo lesions already existing as a pre-nevus lesion.[2]
Histopathology
Melanomas may show the following histological types: spindle cell melanoma (9.0%), mixed cell melanoma (86.0%), and epithelioid cell melanoma (5.0%). [6] When staining cells on pathology, S-100 is the most sensitive for picking up melanocytic lesions. More specific markers for melanoma include HMB-45, tyrosinase, and MART-1/Melan-A. [7]
History and Physical
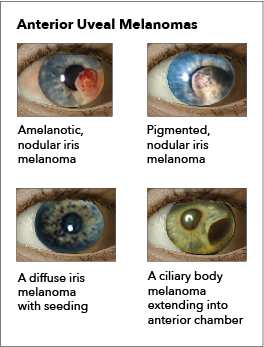
The clinical presentation of ocular melanomas can have a spectrum of symptoms and may depend upon the location of the malignancy. Common symptoms include blurry vision, visual field defect, flashing lights, redness, irritation, pain, and pressure-like sensation. Choroidal tumors can cause retinal detachment based on the shape of the malignancy. Conjunctival melanomas can present as lumps, pigmented lumps, and usually with less pain and irritation. Ciliary body tumors can cause lens displacement and disturbances in accommodation. Iris melanomas can arise from a previous lesion or a new spot, which can distort the pupil, cause cataracts, and even cause glaucoma. [8]
Evaluation
The basic evaluation of an eye malignancy includes fundoscopic examination, fundus photography, and ocular ultrasound. Depending on the location of the malignancy, various imaging modalities are used. If the orbit is involved, CT or MRI is the first step. If lymphatic involvement is suspected, then a PET scan is warranted, along with an MRI of the head and neck, and fine-needle aspiration biopsy is needed. [9] Newer development in ocular oncology with more invasive methods, such as fluorescein angiography (FA) and indocyanine green angiography (ICGA), have been used to help differentiate melanoma from underlying pathology, to assess for secondary neovascularization or ischemia, or to further visualize lesions obscured by media opacity. More recently, optical coherence tomography and optical coherence tomography angiography have made it possible to non-invasively image superficial and deep structures of the retina and choroid, which provides more detail than a simple clinical exam. [10]
Treatment / Management
Depending on the size of the melanoma, various treatment modalities are used. In uveal melanoma, options include the following:
- Plaque radiation therapy
- Particle beam radiotherapy
- Transpupillary thermotherapy
- Laser photocoagulation
- Gamma knife stereotactic radiosurgery
- Local surgical resection
- Enucleation
Advances aimed at vision and eye preservation have been made with techniques such as trans retinal endoresection and transscleral resection.
Studies show that there may be 0% to 15% response rates from using chemotherapeutic agents as used in cutaneous melanoma. Chemotherapy agents that have been used are dacarbazine, temozolomide, cisplatin, treosulfan, and fotemustine. [11]
Iris melanomas can be usually treated with surgical resection. Larger iris melanomas that are not able to be surgically resected will be treated with plaque therapy or enucleation.
Small and medium-sized choroidal tumors are mostly treated by radiation therapy, while larger advanced tumors are treated by enucleation or orbital exenteration when there is spread into the orbit.
Effective treatment regimens for conjunctival melanoma include local excision with adjuvant chemotherapy (mitomycin). [8]
Differential Diagnosis
Clinically, various diseases may present similarly to ocular melanoma.
Choroidal body: Choroidal nevus, choroidal hemangioma, osteoma; hemorrhagic conditions like age-related macular degeneration (AMD) and hemorrhagic choroidal detachment
Iris: Iris nevus, iris foreign body, peripheral anterior synechia, iris pigment epithelial cyst, iris stromal cyst, melanocytoma, iris atrophy, and Cogan-Reese syndrome
Retina: Congenital retinal pigment epithelium hypertrophy and retinal pigment epithelium adenocarcinoma; and inflammatory lesions like posterior scleritis
Various: hemangiomas, sarcoid nodule, metastatic tumor, benign nevus, congenital hypertrophy, intraocular foreign body granuloma, Fuchs adenoma [12]
Radiation Oncology
Brachytherapy involves suturing a plaque to the sclera to deliver focal radiation to the tumor. It is the most frequently employed modality in the U.S. Iodine-125 is the preferred isotope in the U.S. and emits gamma radiation, which penetrates deeply into tumors. Following treatment, a regular ophthalmologic examination should be performed to monitor for complications, including radiation-induced retinopathy, cataracts, neovascular glaucoma, and macular edema, which can develop up to 5 years after therapy. The use of intravitreal anti-vascular endothelial growth factor (VEGF) after brachytherapy has been shown to reduce or delay the rate of macular edema and vision loss. [11]
Staging
At initial diagnosis, the following tests are performed:
- Alkaline phosphatase
- Glutamic-oxaloacetic transaminase
- Lactate dehydrogenase
- Gamma-glutamyl transpeptidase
- CT abdomen and pelvis with contrast
- CT chest with contrast
- CT soft tissue neck with contrast
- Brain MRI
- Liver ultrasound
- Liver MRI with contrast
When the tumor is removed, the following should be noted:
A missing copy of chromosome 3 (monosomy 3) is seen in about 50% of uveal melanoma: this is associated with a higher risk of the tumor metastasizing. Tests are now available by sending a sample of the tumor for testing. Based on the genetic results and using patient information (age, gender, size of the tumor, histology, etc.), the risk of developing metastases is calculated.
Prognosis
Several clinical and histopathological variables of primary uveal melanoma were found to be associated with metastatic death. These include increasing patient age, increasing tumor size (tumor elevation and independently tumor diameter), ciliary body involvement, extraocular extension, epithelioid cell type, lymphocytic infiltration, presence of fibrovascular loops and several biomarkers, including human leukocyte antigen (HLA) molecules, and others, by immunohistochemical methods[13]
Approximately 50% of patients with ocular melanoma will develop metastases by 10 to 15 years after diagnosis. A small percentage of people will develop metastases even later, i.e. 20-25 years after their initial diagnosis. Metastatic disease is universally fatal. This 50% mortality rate is unchanged despite treatment advances in treating the primary eye tumor. More research is needed urgently to improve patient outcomes. [8]
Complications
Post radiation complications include radiation-induced retinopathy, cataracts, neovascular glaucoma and macular edema, which can develop up to 5 years after therapy.
Consultations
- Opthalmology
- Oncology
- Radiation oncology
Deterrence and Patient Education
Monitoring for metastasis should be performed regularly with emphasis on compliance with follow up visits, regardless of stage and treatment. Patients should have dilated fundal examination, liver function test, alkaline phosphatase, LDH, and CT or right upper quadrant ultrasound to assess for liver metastasis. Frequency depends on the stage of the tumor, and those that have a high risk of metastasis should be monitored as frequently as 3-4 months. [14]
Enhancing Healthcare Team Outcomes
Ocular melanoma is a serious and lethal clinical malignancy with a poor prognosis when it spreads. Close attention needs to be paid attention to when patients present with benign lesions in their uveal tract from their iris to the choroid. An interprofessional team can be helpful for proper assessment and treatment. Optometrists and primary care providers may first see the lesion. A full ophthalmologic evaluation must be done and then staging based on size with ophthalmology. Patients should have intervention done via enucleation or other tactics with neurosurgery. Medical and radiation oncologists may be consulted. Ophthalmology and oncology nurses are involved in treatment, monitor patients, and assist in team coordination and followup. Oncologic pharmacists review medications, verify doses and check for drug-drug interactions. Pharmacists and nurses help with patient and family education. [Level 5] Close follow up with repeat PET scans directed by the oncologist should be done diligently. An interprofessional approach will result in the best outcomes. [Level 5]