Pancreas Imaging
- Article Author:
- Kyle Stevens
- Article Editor:
- Christopher Lisanti
- Updated:
- 4/20/2020 6:16:46 AM
- For CME on this topic:
- Pancreas Imaging CME
- PubMed Link:
- Pancreas Imaging
Introduction
The pancreas is an abdominal organ possessing both endocrine and exocrine functions. It produces a variety of hormones that mostly pertain to regulating blood sugar levels. As an exocrine gland, it secretes pancreatic fluid that contains bicarbonate and digestive enzymes. Commonly, there are a few broad categories of diseases that affect the pancreas: pancreatitis, pancreatic insufficiency, cystic lesions of the pancreas, and pancreatic tumors.
Pancreatitis is a generalized inflammation of the pancreas due to activation of digestive enzymes produced by the pancreas while still inside the organ. Acutely, this can result from gallstones, alcohol-misuse, or hypertriglyceridemia. Gallstones are the etiology of the majority of acute pancreatitis cases, 40 to 70%,[1] while alcohol misuse accounts for 25 to 35%.[2] Pancreatitis typically presents as an acute onset of epigastric pain that may radiate through to the back. Nausea and vomiting frequently accompany abdominal pain. The revised Atlanta classification separates acute pancreatitis into two subtypes: interstitial edematous pancreatitis and necrotizing pancreatitis. The severity further classifies acute pancreatitis into mild, moderately severe, or severe, based on the absence of organ failure, the presence of transient organ failure, or persistent organ failure, respectively.[3] The mortality rate from acute pancreatitis is approximately 5%, while those with necrotizing pancreatitis see a higher rate at 17%.[4] Chronic pancreatitis can occur in patients with alcohol use disorder, in which scarring of the gland prevents it from functioning properly.
Pancreatic insufficiency is the result when the pancreas is unable to produce enough digestive enzymes to break down food in the digestive tract. This condition is typically a deficiency in the exocrine function of the gland that can be caused by a variety of disease processes; most commonly cystic fibrosis in children and chronic pancreatitis in adults.
Pancreatic exocrine cancer is one of the leading causes of cancer-related deaths in the United States, trailing only behind lung, colorectal, and breast. It also ranks among the most lethal cancers, with a one-year survival rate of 20% and a five-year survival rate of only 5%.[5] The lethality is mostly due to the insidious onset of the malignancy, with symptoms (e.g., jaundice, weight loss, and vague subacute epigastric pain) not presenting until late in the course of illness. Frequently, at the time of diagnosis, the lesion is inoperable due to extension into nearby structures. Over one-third of the tumors are Stage IV upon identification, and less than 20% of these cancers are candidates for surgical resection.[6][7] The most common malignancy is a ductal adenocarcinoma involving the exocrine glands; the majority of these tumors get discovered in the head of the pancreas.
Pancreatic neuroendocrine tumors (NETs) are malignancies that form in the endocrine tissue of the pancreas. Also known as islet cell tumors, these are rare tumors occurring in approximately 1 in every 100000 people, and only 1% of all pancreatic tumors are NETs.[8] NETs can result in the overproduction and secretion of pancreatic hormones, including insulin, gastrin, glucagon, and vasoactive intestinal peptide (VIP), resulting in specific clinical syndromes on presentation.
Cystic lesions of the pancreas are a relatively common incidental finding on body imaging, with prevalence in the general population ranging from 2.4 to 24%. Classification of these cysts is important as they can either be true cysts, pseudocysts (usually related to pancreatitis), or related to benign or malignant neoplasms. Radiological imaging has historically helped distinguish the etiology of the cystic lesions in 75 to 90% of presentations.[9] True epithelial cysts are rare in the general population and are classically only associated with cystic fibrosis, von Hippel-Lindau disease, an autosomal dominant polycystic kidney disease. Pseudocysts, related to pancreatitis or trauma, are the most common cystic lesion identified in the pancreas. Approximately 20 to 40% of patients with chronic pancreatitis develop pseudocysts, while only 2-3% of those with acute pancreatitis will develop them.[9] Cysts associated with malignancy may rarely occur with exocrine tumors, occasionally with endocrine tumors, or in isolation. Cystic neoplasms include intraductal papillary mucinous neoplasms, mucinous cystic neoplasms, and serous cystadenomas.
Anatomy
The anatomy of the pancreas and nearby structures are of particular importance for understanding gallstone pancreatitis and surgical contraindications to pancreatic cancer.
The common bile duct enters the pancreas and merges with the main pancreatic duct, just prior to the ampulla of Vater, where they both empty into the second part of the duodenum through the major duodenal papilla. In gallstone pancreatitis, a gallstone that escaped the gallbladder moves down the common bile duct, entering the pancreas. The gallstone can become stuck at the ampulla of Vater, blocking both the common bile duct and the main pancreatic duct from being able to empty into the duodenum. This blockage will cause a back-up of both bile and pancreatic juice, resulting in gallstone pancreatitis.
The anatomy of both the pancreas and surrounding structures is critical for understanding the surgical indications and contraindications for pancreatic cancer. The head of the pancreas resides in the “C-loop” of the duodenum, with the uncinate process curling back and behind the superior mesenteric artery and vein. The pancreatic neck crosses over the inferior vena cava and aorta near the celiac trunk, while the tail continues to traverse to the left side of the abdomen, along with the splenic artery, terminating at or near the splenic hilum. The stomach sits in front of the pancreas. All of these nearby structures, particularly the celiac axis and superior mesenteric vessels near the head of the pancreas can become involved in a rapidly enlarging pancreatic malignancy complicating the surgical approach or making it inoperable.
Plain Films
The use of plain radiographs is extremely limited in the evaluation of the pancreas. Although not diagnostic, calcifications of the pancreas may be visible on abdominal radiographs in the setting of chronic pancreatitis.
Computed Tomography (CT) vs. Magnetic Resonance Imaging (MRI) vs. Ultrasound
A few general principles common to imaging in various body parts are essential when deciding which of these three cross-sectional modalities to use, and the relative strengths and weaknesses of each. In general, CT and MRI have large fields-of-view and are both very effective in identifying local abnormalities along with regional or more distant involvement. Ultrasound has a limited field-of-view and can be subject to substantial limitations by body habitus and overlying gas.
CT and MR are better than ultrasound for characterizing solid lesions due to the advantage of administering intravenous contrast material, and differences in signal intensity seen on MRI. On the other hand, cystic lesions can be detected with all three modalities, but are better characterized by ultrasound and even better by MRI.
Computed Tomography
Computed tomography (CT) is the backbone of pancreatic imaging. Intravenous contrast is nearly always indicated with multi-phase enhanced imaging being optimal for identifying and characterizing pancreatic masses or complications from pancreatitis. Multi-phase pancreas protocols will usually include an unenhanced sequence, a ‘pancreas phase’ or late-arterial phase as well as portal venous phase sequences.
In acute pancreatitis, intravenous contrast-enhanced CT will show focal or diffuse enlargement of the pancreatic parenchyma due to interstitial edema, with heterogeneous enhancement. The pancreatic margins will be ill-defined due to inflammation, and peripancreatic fat stranding may also be present. An obstructing gallstone may or may not be visible if that is the cause of pancreatitis. Complications may be seen acutely or delayed, including necrosis, hemorrhage, vascular complications, pancreatic pseudocysts, and abscess formation. Areas that lack contrast-enhancement are diagnostic of pancreatic necrosis.[10] Visualization of necrotic pancreatitis is best between 48 to 72 hours after onset; therefore, early scanning in acute pancreatitis might under-call patients with necrosis. Initial imaging is recommended to be performed 5 to 7 days after hospital admission.[3] In cases of infected necrosis, gas bubbles may be apparent, which is pathognomonic for emphysematous pancreatitis. Hemorrhagic pancreatitis can present radiographically as a fluid of high attenuation on unenhanced CT, with the attenuation decreasing as the blood ages.[11] Vascular complications include thrombosis of the portal vein, which are identifiable as either no enhancement or a filling defect of the portal vein. An additional vascular complication is aneurysm formation of any of the peripancreatic arteries.
Regarding chronic pancreatitis, findings may include a dilated main pancreatic duct, atrophy of the pancreatic parenchyma, and pancreatic calcifications. While pancreatic atrophy occurs in approximately 54% of patients with chronic pancreatitis, this is not a specific finding and can also occur with normal aging.[12] Pancreatic pseudocysts will typically present as a unilocular fluid collection without enhancing solid components.[9]
Nearly nine out of ten cystic fibrosis patients experience pancreatic exocrine gland insufficiency due to ductal obstruction and subsequent fibrosis and fatty replacement. This blockage can cause acute pancreatitis when there is still residual pancreatic exocrine function. CT imaging can reveal fatty replacement of the pancreas, which appears as areas of fat (low) attenuation. Eventually, the continuing fatty replacement can lead to complete pancreatic lipomatosis, typically before the patient reaches 20 years of age.[13] Calcifications and small pancreatic cysts may also be present. True epithelial cysts will generally have no internal septa or enhancement post-intravenous contrast administration.
When evaluating pancreatic exocrine tumors, the ‘pancreas phase’ sequence best accentuates the attenuation difference between the malignancy and the healthy pancreatic tissue,[14] due to the tumor’s hypovascularity. The masses will appear on CT as ill-defined. The lesion itself is usually hypodense throughout arterial phase sequences,[15] although up to 10% of tumors, especially those less than 2 cm, will be isodense compared to the normal pancreatic parenchyma.[16] Associated signs, such as pancreatic duct cutoff and the double duct sign may also be visible. The overall reported sensitivity of CT in detecting pancreatic cancer is 89 to 97%,[7] with a positive predictive value of 89% in predicting the surgical resectability of the tumor.[16]
Multiphase, contrast-enhanced CT is also the ideal imaging technique for evaluating pancreatic neuroendocrine tumors. NETs are highly vascular tumors that will enhance during the arterial or pancreatic phase. Washout should be noted during the portal venous phase sequencing.[11] Smaller tumors are well-circumscribed, homogeneous masses on CT; however, larger NETs can have a variety of appearances due to necrosis, cystic changes, or hemorrhage. Isolated cystic neoplasms are potentially visible via CT; however, characterization is usually more effective with magnetic resonance imaging. Intraductal papillary mucinous neoplasms (IPMN) may show pancreatic ductal dilation and/or cystic lesion. The cystic lesions are typically hypoattenuating and heterogeneous. Mucinous cystic neoplasms will present as hypoattenuating as well but will often display a thick enhancing wall on delayed imaging. Whereas, serous cystadenomas may have a central scar with ‘sunburst calcifications’ extending out from the central focus in up to 30% of cases.[9]
Magnetic Resonance
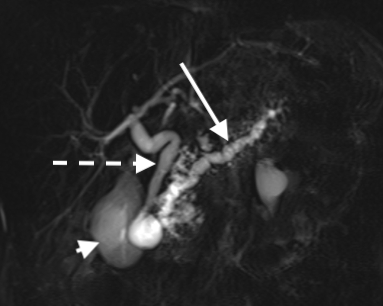
Although CT is the initial modality for diagnosis of pancreatic abnormalities, MRI is beneficial for characterizing cystic lesions, and characterizing and even identifying some solid tumors, particularly smaller ones. Additionally, magnetic resonance cholangiopancreatography (MRCP) can better visualize the pancreatic and biliary ducts, and demonstrate any communication or obstruction related to a lesion. Typical pancreatic MR protocol includes T1- and T2-weighted images with multiphasic imaging after intravenous gadolinium administration.
Pancreatitis evaluation in the acute setting is nearly always with CT; however, MRI is comparable to CT and can be useful in some complicated cases or in patients who cannot receive iodinated contrast. Fat-suppressed T2-weighted sequences demonstrate greater differentiation between pancreatic and peripancreatic tissues while making any peripancreatic inflammation much more conspicuous. T2-weighted images can also help distinguish simple appearing pseudocysts from more internally complex walled-off necrosis. MRCP can demonstrate the pancreatic and bile ducts along with gallstone detection with higher sensitivity than CT. Fat-suppressed, T1 weighted images will reveal focal or diffuse enlargement of the pancreatic parenchyma with ill-defined borders, similar to findings on CT. The inflamed pancreatic parenchyma will be hypointense when compared to the normal pancreas or the liver on T1 and hyperintense on T2, due to inflammation and edema. Necrosis will appear hypointense on T2-weighted images and hyperintense when liquified.[17] Hemorrhage can be visualized via fat-suppressed T1 and T2-weighted images, but gradient-recalled echo (GRE) sequences are also extremely sensitive to the magnetic properties of hemoglobin.[11] Gadolinium-enhanced T1-weighted images will be best for the assessment of necrosis and the extent of inflammation.
MRCP is increasingly in use for the diagnosis of chronic pancreatitis due to its ability to visualize pancreatic duct obstruction and dilation; however, the typical calcifications in chronic pancreatitis are not visualized as well as on CT. Pancreatic atrophy can be seen as irregular margins of the pancreas and decreased T1-weighted signal with delayed enhancement post-gadolinium injection. Secretin-enhanced MRCP (sMRCP) provides better visualization of the main pancreatic duct and its branches. Pancreatic duct flow rates can also be evaluated with sMRCP.[12] Pseudocysts will present as hyperintense lesions on T2-weighted images, while pseudocysts containing blood will have increased signal on T1-weighted sequences.[9]
MR can help differentiate pancreatic lipomatosis from fibrotic changes in cystic fibrosis patients. Fibrotic replacement of the pancreas will appear as hypointense areas on T1- and T2-weighted imaging, while fatty changes will reveal the opposite: hyperintensity on both T1- and T2-weighted sequences.[13]
Pancreatic adenocarcinomas appear hypointense on T1-weighted images vis-a-vis the normally bright appearance of the healthy pancreas. There is less utility of T2-weighted images as the signal is similar to the normal pancreas. Diffusion-weighted images can show restricted diffusion of adenocarcinomas, but restricted diffusion is not specific to carcinomas as it can also present in pancreatitis.[16] However, utilizing diffusion gradients with lower b-values, correlating with lower amplitudes, shorter durations, and shorter intervals between gradient pulses, may accentuate subtle T2-weighted signal abnormalities. MRCP may reveal ductal obstruction in the area of a suspected small mass. The double duct sign of simultaneous dilation of the common bile and pancreatic ducts may also be visible.
Pancreatic NETs appear on MRI as hypointense on T1- and hyperintense on T2-weighted images with hyperenhancement after gadolinium administration on arterial or pancreatic phase timing due to their hypervascular nature. Large islet cell tumors may have areas of cystic or necrotic nonenhancement.[18] MRI can further characterize cystic neoplasms. IPMNs should show either main pancreatic ductal dilation (diffuse or segmental) or a multi-cystic lobulated mass. Mucinous cystic neoplasms appear congruent with simple cystic fluid collections that may have fluid-fluid levels on T2-weighted images. Postcontrast T1-weighted sequences may show enhancing mural nodules within the lesion. The main pancreatic duct is usually not involved. Serous cystadenoma will typically reveal numerous small cysts that are hyperintense on T2-images. Thin septa may enhance, and if a central scar is present, it will enhance on delayed imaging.[9]
Lastly, in cases of biliary ductal dilation, MRCP can help differentiate an obstructing gallstone (no enhancement and dark on T2) or intraluminal mass (enhancing mass without dark T2-weighted signal).
Ultrasonography
Transabdominal ultrasound’s main role in acute pancreatitis is to identify any gallstones and/or choledocholithiasis. Otherwise, ultrasound has limited use in significant measure due to difficult visualization of the pancreas often due to overlying bowel gas.[19] The inflamed pancreas will appear hypoechoic and diffusely enlarged due to edema. Anechoic fluid collections may be present in severe disease, representing peripancreatic fluid.
On the other hand, endoscopic ultrasound (EUS) may be useful in the setting of chronic pancreatitis evaluation. Features of the pancreatic parenchyma include hyperechoic foci/strands due to fibrosis and cysts. Dilation of the main pancreatic duct over 3 mm may be visible with areas of focal narrowing and hyperechoic margins representing periductal fibrosis. Side branches of the main duct may also be dilated with a diameter over 1 mm. EUS is highly sensitive for detecting changes in the normal pancreatic architecture; however, limitations are mainly due to operator variability and its more invasive nature.[12] Pseudocysts manifest as unilocular, anechoic lesions with thin-walls.
Pancreatic lipomatosis, in the setting of cystic fibrosis, classically shows increased pancreatic echogenicity on ultrasonography. The characteristic lobular architecture of the pancreas may also appear diminished.[13]
Transabdominal ultrasound is not an uncommon initial imaging modality for a patient with jaundice, epigastric pain and/or weight loss as ultrasound is highly sensitive for detecting obstruction of the biliary tract and subsequent dilation. If these symptoms are due to a pancreatic carcinoma, the mass usually appears as an ill-defined and hypoechoic, hypovascular structure with or without ductal dilation.[16]
Ultrasound’s place in the evaluation of pancreatic NETs is uncertain. However, these well-circumscribed, hypoechoic, and hypervascular lesions can sometimes be seen on ultrasound, even if their morphology is nonspecific. EUS can be helpful in the setting of both exocrine and endocrine tumors, as biopsy via FNA can be performed during the ultrasound. Differentiating various cystic lesions via EUS alone may be challenging. However, when using EUS in conjuncture with fine-needle aspiration (FNA), it is the most effective means of classifying cystic lesions of the pancreas, with specificity greater than 90%.[9] Morphologically, mucinous cystic neoplasms may be unique in demonstrating internal septa with differing thicknesses and containing hyperechoic internal debris. Microcystic serous cystadenomas have features that are highly specific for its etiology: multiple demarcated anechoic cystic lesions separated by thin septa resulting in a ‘honeycomb’ appearance that is not associated with the pancreatic ducts.[9] Alternatively, macrocystic serous cystadenomas may look very similar in appearance to IPMNs and mucinous cystic neoplasms via ultrasonography.
Nuclear Medicine
In patients with NETs, nuclear medicine studies may be necessary for further staging and characterization of the lesion. Historically, octreotide scans have been performed, in which octreotide (a somatostatin analog) is linked to the tracer indium-111, and then injected before imaging. This modality later evolved into indium-111 octreotide getting coupled with single-photon emission computed tomography (SPECT). More recently, somatostatin receptor (SSTR) positron emission tomography (PET) is being increasingly utilized due to improved sensitivity for detecting small lesions, decreased radiation dose, and its ability to quantify uptake. SSTR PET uses Ga-DOTATATE and Ga-DOTATOC tracers, which both have high affinities for subtype 2 SSTRs.[20] SSTR PET can be coupled with MRI or CT for greater spatial resolution.
Clinical Significance
The differential of pancreatic disease processes is not only challenging, but it is one that can demonstrate significant morbidity and mortality in the population. Understanding the various imaging modalities available and when each is appropriate is of critical importance. CT and MR provide an excellent spatial resolution of the pancreatic anatomy and are most often the modalities of choice when considering the pancreas. EUS-FNA is highly specific and valuable in diagnosing pancreatic cystic lesions.
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)