Peritoneal Cancer
- Article Author:
- Ayesha Anwar
- Article Editor:
- Anup Kasi
- Updated:
- 9/3/2020 8:38:20 AM
- For CME on this topic:
- Peritoneal Cancer CME
- PubMed Link:
- Peritoneal Cancer
Introduction
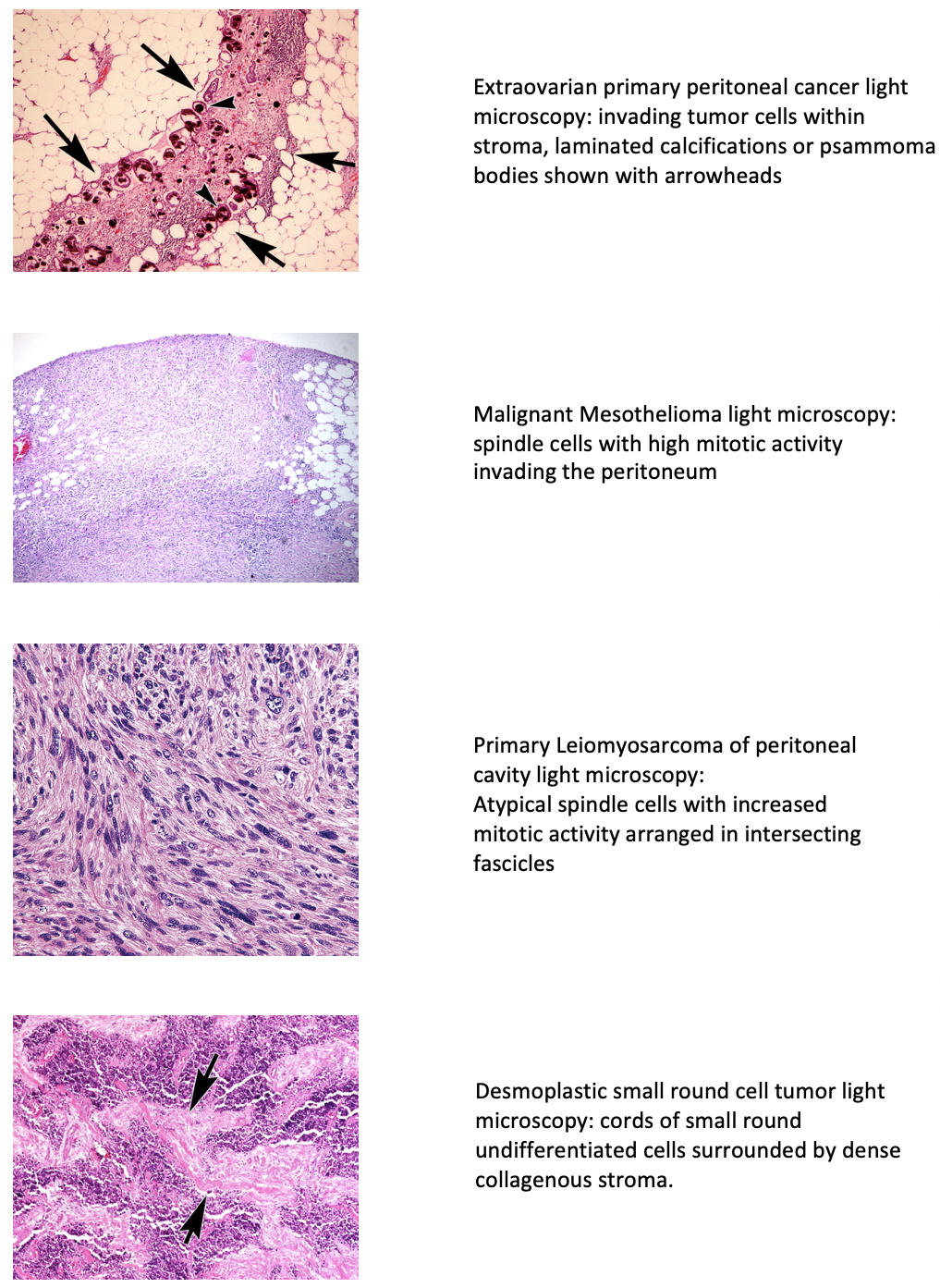
The invasion of the serous membrane lining the abdominal cavity, viscera, and coelom in amniotes by malignant cells is called peritoneal surface malignancy or peritoneal cancer (PC). It is divided into primary and secondary types. The de novo origin of cancer in the mesothelium of the abdomen causes primary mesothelioma. In contrast, the dissemination of tumor cells in the peritoneal cavity from other sites results in secondary peritoneal cancer. Primary cancer has been classified based on its histology by investigators. Extraovarian primary peritoneal carcinoma (EOPPC), serous surface papillary carcinoma, papillary serous carcinoma of the peritoneum, extra ovarian Mullerian adenocarcinoma, and normal-sized ovarian carcinoma syndrome all are different terms used for the first type.[1]
Other types include malignant mesothelioma (MPM), multicystic mesothelioma, leiomyosarcomas, leiomyomatosis peritonealis disseminata, and desmoplastic small round cells tumor (DSRCT).[2] Swerdlow first reported EOPPC as ‘Mesothelioma of pelvic peritoneum’ in a case report published in 1959.[3] It behaves similarly to serous ovarian cancer with little or no involvement of ovaries. All the types have variable histological features but are alike in their presentation, diagnostic evaluation, and treatment methods. Secondary or metastatic peritoneal carcinomatosis arises commonly from primitive malignancies involving gastrointestinal and gynecological structures. The metastasis occurs via transcoelomic, vascular, or lymphatic routes. It was first described in 1931 as a local spread from ovarian cancer.[4]
Primary cancer is classed as stage III or IV and metastasis as stage IV. The vague clinical presentation is responsible for late diagnosis and an overall decrease in survival. The surgical resection and intraperitoneal chemotherapy form hallmarks for disease eradication. However, a better understanding of peritoneum physiology and tumor seeding pathways combined with advancement in technologies has led to the development of effective treatment therapies. In the absence of extensive systemic disease, locoregional control of the disease can provide a promising role in the management of this late-stage cancer.
Etiology
Primary peritoneal cancer is idiopathic cancer arising from peritoneal layers of the abdominal cavity. Its subtype, EOPPC, resembles serous ovarian carcinoma and occurs exclusively in women (mean age, 56-62 years). There are just a few reports of its occurrence in males. It can be caused by germline mutations in the BRCA 1 gene which has been reported in 17.6% of cases.[1] Thus, in any patient with familial breast cancer, serous peritoneal cancer should be excluded. Malignant peritoneal mesothelioma is an aggressive tumor caused by asbestos exposure in 33% to 50% of cases and occurs in older males (60 years and older).[5]
Disseminated peritoneal leiomyomatosis is associated with a high estrogenic state in postmenopausal women. Leiomyosarcoma is a secondary tumor occurring in Li Fraumeni syndrome along with retinoblastoma. Desmoplastic round small cell tumor occurs in adolescents (median age 19 years) and 85% in Caucasians. It is caused by [t(11;22)(p13;q12)] translocation.[6]
Secondary peritoneal carcinomatosis is commonly caused by invading malignant cells from tumors involving the stomach, colon, pancreas, gall bladder, appendix, breast, uterus, ovary, and lungs. The peritoneal involvement in appendiceal cancer is called pseudomyxoma peritonei (PMP). It is successfully managed and results in a lifetime without relapse. The metastasis from ovarian, gastric, and colorectal malignancies is associated with increased chances of recurrence and fatality, and they are also the three most common etiologies of metastatic spread in the peritoneum.
Epidemiology
Peritoneal tumors are rare advanced malignancies of the human body. The age-adjusted incidence rate of primary peritoneal cancer is 6.78 per million.[7] The rate is highest among white people and lowest among black. The most common histological type of primary peritoneal cancer is serous carcinoma of the peritoneum and accounts for 10% of cancers occurring in the pelvis.[8] Malignant mesothelioma is a less common and highly lethal malignancy. Pleural mesothelioma comprises the most number of cases, and peritoneal mesothelioma (MPM) follows it. MPM occurs in 10% to 15% of cases of mesothelioma.[9] About 50% of leiomyosarcomas occur in the retroperitoneum.[10]
Peritoneal metastasis is the most common malignant process occurring in the peritoneal cavity. In ovarian cancers, peritoneal metastasis is present in 75% of cases at the time of diagnosis.[11] In a study done, it is reported to occur synchronously with the primary tumor in 55% of cases and on follow-up in 45% of cases of nongynecological malignancies.[12] Colorectal tumors are associated with the peritoneal spread at the time of diagnosis in 5% to 10% of cases and have metachronous malignant proliferation in 20$% to 50% of cases.[13] Peritoneal dissemination by gastric cancers is found in 14% of cases at initial presentation.[14] In addition to viscera within the peritoneal cavity, metastasis in the peritoneum also results from extra-abdominal malignancies in 9% of cases.[15] This includes breast (40.8%), lung (25.6%), and melanoma (9.3%) as the most common sites.
Pathophysiology
The peritoneum is the serous membrane lining the abdomen, which supports the abdominal viscera and provides a conduit for blood, lymph, and nerve conduction. It consists of two layers; the parietal peritoneum, attached with the abdominal wall, and the visceral peritoneum surrounding the organs. The space between the bilaminar layers is the abdominal cavity or coelom and contains the peritoneal fluid, which encloses abdominal organs and provides lubrication for peristaltic movements. Its volume is around 100ml.[16]
The peritoneum is the largest dynamic membrane that can adapt according to different pathologies. Histologically, it consists of mesothelium and submesothelial connective tissue, separated by a thin basement membrane called basal lamina comprising of collagen IV and laminin. The elastic matrix is made of collagen I, III, and cells such as fibroblasts, adipocytes, and macrophages. It also hosts lymphatics and blood vessels. The mesodermal layer is derived from mesoderm and has both the characteristics of epithelial and mesenchymal cells. It acts as the first line of defense due to the tight junctions among the cells and also expresses cytokeratin, fibronectin, and other markers. It binds tumor cells with peritoneum. The peritoneal deposits occur at sites of immune cell aggregates, ‘milky spots’ named by Ranvier, and contain mesothelial cells and blood vessels. The submesothelial stroma causes adhesion to cancer cells via integrins and hence, penetration into the peritoneum.[17]
The carcinogenesis of peritoneal cancers can be explained by ‘seed and soil theory’ given by Stephen Paget. It describes how a malignant tumor gives up cells (seeds) that travel in all directions but can only survive and multiply at tumor accepting localizations (soil).[18][19] It explains the predilection of colorectal, ovarian, and gastric tumor cells for peritoneum. Organ-specific metastasis is due to molecular interaction and compatibility between receptors on malignant cells and ligands on host cells. This was explained by Sugarbaker in 1979.[20]
The primary spread from the tumor is due to the extensive intramural growth beyond the serosal layers. The secondary seeding occurs in surgical tumor resections that result in the spillage of malignant cells. The first step is the detachment of cancer cells from the primary due to the down-regulation of intracellular adhesion molecules called E- Cadherin.[21] Then, tumors cells enter the bloodstream, lymphatics, or intracoelomic route to be carried to distant specific sites, and vascular factors present in the peritoneal fluid promote their growth during transport. The process of metastasis, including adhesion, degradation, migration, angiogenesis, and immune evasion, follows.[17]
The adhesion molecules on mesothelial cells like intercellular adhesion molecule 1 (ICAM-1), and vascular adhesion molecule 1 (VCAM-1) interact with tumor cells’ receptors like CD44 and cytokines such as tumor necrosis alpha (TNFa), interleukin- 1beta, and interleukin-1gamma are released. Hence, the basement membrane is exposed. CD44 has a role in metastasis of colorectal and ovarian cancer.[22] In trans-lymphatic metastasis, cells enter the lymph capillaries at milky spots or lymphatic stomata. It is seen in PMP. The cells then invade the submesothelial stroma, where hepatocyte growth factor (HPF) binds to its tyrosine kinase receptor and start the growth of the tumor. This is mediated by c-MET protoncogene. The fibroblasts and macrophages present within the matrix secrete metalloproteinases (MTP) and degrade the peritoneal-blood border in the stroma. This results in the progression of metastasis. Finally, the growth of tumors takes place by the production of growth factors like IGF-1 and EGFR. To sustain this exponential production of cells, newer blood vessels are formed through the production of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor 1 (HIF-1).
EOPPC pathogenesis can be explained by the theory of retention of ‘differentiating potential’ by mesothelial cells of peritoneum. The cells undergo a malignant transformation called ‘Mullerian metaplasia’.[23] The same embryonal mesoderm forms the germinal epithelium of ovaries and mesothelial cells of the peritoneum, which explains the resemblance of the two tumors. The oncogenic stimulus is the loss of heterozygosity at p53 or BRCA-1 loci or the overexpression of Her-2/neu.[24] Its pathogenesis is also explained with the multifocal origin of the tumor. The process of nonrandom X chromosome inactivation follows a consistent pattern, that indicates the unifocal origin of tumors. However, different patterns of inactivation result in tumors that have independent sites of development. Gu et al. did a study regarding the clonality of peritoneal and ovarian cancer. 54% of patients showed nonrandom inactivation, and most had different patterns.[25]
Histologically, psammoma bodies are characteristic of this tumor.
Asbestos exposure is linked to the development of MPM. Other risk factors are talc, mica, erionite, volcanic ash, radiation, and chronic peritonitis. Asbestos inhalation is responsible for causing cellular damage, which leads to carcinogenesis. The following essential processes explain it:
- Asbestos in the body produces reactive oxygen species damaging DNA.
- Asbestos fibers either physically disrupt the cell cycle genes by entering mesothelial cells or induce inflammation and tumorigenesis by releasing cytokines and growth factors from macrophages and mesothelial cells. Epigenetic modifiers like BRCA-1 associated protein-1 (BAP-1) and cyclin-dependent kinase inhibitor 2A/ alternative reading frame (CDKN2A/ARF) genes are specific for mesothelial cell transformation in MPM. High-mobility group box 1(HMGB1) produced by mesothelial cells causes necrosis, inflammation, and carcinogenesis. TNFa released by macrophages causes activation of nuclear factor-kb and promotes cell survival.[26]
- Lastly, the large surface area of asbestos renders it to absorb more carcinogens, thus promoting malignancy.
Histologically, they are divided into epithelial, sarcomatous, and bimorphic types.
Leiomyosarcomas show variable smooth muscle actin and eosinophilic spindle cells. It is caused by a deletion in retinoblastoma gene1 (RB1) 10q and PTEN 13q. The mutations at TP53 also add to the risk.[27]
Desmoplastic small round cell tumor, a small round blue cell tumor, is associated with Ewing tumor family chromosomal translocation t(11;22)(p13;q12), causing the formation of EWSR1-WT1 fusion oncogene that result in tumor development.[28]
History and Physical
Clinical presentation in peritoneal cancer is variable depending on the extent of involvement. It is usually diagnosed in late stages due to the vague symptomatology. EOPPC has an indistinguishable presentation from an epithelial ovarian cancer. The Gynecologic Oncology Group has defined the following criterion for EOPPC diagnosis:
- Both ovaries should be of normal size, and the enlargement is benign.
- The surface area of malignant involvement of extra-ovarian sites should be larger than either of the ovaries.
- There should be no malignancy in either ovaries or the tumor in serosa, and cortex of size less than 5X5 mm can be present.
- The tumor in the extra-ovarian site should be serosal both histologically and cytologically.[1]
All the peritoneal carcinomas present with non-specific symptoms like abdominal bloating, distension, nausea, indigestion, anorexia, weight loss, fatigue, constipation, abdominal or back pain. The most common symptoms for presentation are abdominal distension and pain, while usual signs are palpable abdominal mass and ascites. Non-specific abdominal symptoms and ascites occur in 85% of the patients.[29] Tumor-associated lymphadenopathy causes local mass effects and can even lead to obstruction of superior vena cava. This has been seen primarily in peritoneal malignant mesothelioma. Patients with DSRCT can also present with hematemesis in some cases.[30] The mesothelium involvement in the absence of primary is occasionally seen as an incidental finding in laparotomy or autopsy.
Peritoneal carcinomatosis has characteristic symptoms of the primary tumor itself and non-specific symptoms. The secondary metastatic deposits may range from microscopic involvement to nodules to bulky disease, and this extent of involvement and location determines the symptoms.
The growth in both primary and secondary tumors causes pressure effects resulting in mechanical intestinal obstruction. Such patients present in an emergency with an ‘acute abdomen.’ Bowel obstructions are seen mainly in colorectal cancers in about 20% of cases.[31] In the same study, ascites were reported in 43% of cases of pancreatic cancer.
Evaluation
The ‘hidden’ existence of a malignant tumor in the peritoneum is responsible for increased fatality in patients of PC. Therefore, practical methods for early and timely diagnosis are required to prevent surgeries of unresectable tumors and harm from un-necessary chemotherapy drugs.
Techniques for diagnosing PC are crucial as most tumors are often discovered incidentally during the surgeries. It includes:
CT Scan
CT scan is the foremost modality used in patients presenting with abdominal pain and distension. In some cases, a preliminary test like Ultrasound (USG) is done. The USG is, however, unable to detect malignant granulations less than 2 cm in size. In PC, ultrasonographic features include ascites which is echo-free or have low-level echoes, and hyperechogenic nodules representing cell deposition in the peritoneum. Adhesion of bowel loops, omental matting, and lymphadenopathy can also be seen.[32]
CT scans can detect granulations of size as small as 5mm.[33] In PC, it has a sensitivity of 70% if the lesion is 2 cm, which is further reduced to 28% in the case of tumor size of 5 mm.[31] The CT scan findings are non-specific and similar in both neoplastic and non-neoplastic conditions. Ascites is the most common finding together with contrast-enhanced diffuse or nodular thickening of the peritoneum. Oral and intravenous contrast material is administered, particularly for viewing small peritoneal deposits. It is true for cystic lesions but limits the identification of calcified lesions.[34] In addition, omental cakes, defined as increased density of large cell masses between the bowel and anterior abdominal wall, are also seen.
The CT scan findings in EOPPC are similar together with normal ovaries and the absence of the primary tumor. Chiou et al. reported that common CT scan findings in primary PC are ascites (82%), peritoneal nodules (73%), omental caking (64%), and pelvic mass (36%).[35]
In MPM, the CT scan identifies a solid, heterogeneous mass with irregular margins. It shows ascites in 60-100% of cases. The MPM usually lacks lymph nodes and distant metastasis.[36]
The leiomyosarcomas show a contrast-enhanced heterogeneous solid and cystic mass with septations, necrosis, and calcifications.
The scan in the DSCRT demonstrates a well enhanced lobulated mass comprising of necrosis, hemorrhage, and fibrous components, usually in retrovesical or rectouterine space.
Hence, the CT scan is the primary diagnostic modality and also helps in guiding the interventional radiologists for conducting biopsies and surgeons for performing cytoreductive surgeries.
MRI Scan
Gadolinium (Gd)-enhanced MRI is better than the helical CT scan for visualization of small peritoneal carcinomatosis. The detection sensitivity of MRI is 84% for tumors of all sizes as compared to 54% for CT scans. The sensitivity is further increased for tumors with dimensions less than 1 cm (85-90%).[37] For peritoneal tumors, T1 shows an intermediate signal, T2 high signal, and C+ (Gd) enhancement. The use of MRI in PC has increased over time and is now the imaging modality of choice for diagnosing and staging subcentimeter lesions due to its higher contrast resolution.[34] The histological type is differentiated on biopsy due to resemblance in radiological appearances. Therefore, imaging techniques in PC are used for identification of the following aspects:
- The primary site of metastasis.
- The morphology of the main tumor as solid, cystic, or mixed.
- The quantity of ascites.
- The presence/absence of peritoneal dissemination.
- Diffuse or nodular spread.
- The lymphadenopathy and lymph nodes involved.
- Distant sites of metastasis if present or not.
PET Scan
The mainstay of imaging modalities for PC is CT scans and MRIs. However, small peritoneal implants are not visible by them. In such cases, FDG PET-CT scan (2-[Fluorine 18] fluoro-2-deoxy-D-glucose positron emission tomography) has the potential to improve the detection. It identifies malignant cells due to their increased glucose metabolism. This method helps in early detection, staging, monitoring treatment response, and long-term follow up.[38] The sensitivity of fused PET-CT scan (unenhanced) and PET-MDCT scan (multidetector) ranges between 58% to 100%.[34] In the FDG PET-CT scan, false-negative results occur due to certain tumor cells that do not take up ‘F’ such as mucinous tumors of ovaries or signet ring gastric cancer. In contrast, false-positive results are seen in benign and inflammatory conditions where cells take up ‘F.’ The diffuse or nodular uptake of ‘F’ by cells in the peritoneum can result in detecting certain occult malignancies or metastasis and significantly impact the management of PC.
Invasive Techniques
The resemblance in radiological appearances of neoplastic and non-neoplastic lesions is responsible for establishing the requirement of invasive techniques. For example, the histological type and subtypes of the tumor can only be differentiated on biopsy. In PC, invasive procedures include:
1. Abdominal percutaneous paracentesis.
2. Diagnostic laparoscopy.
1. Paracentesis
Ascitic fluid analysis
In PC, ascites is exudative with high protein (2.5 mg/dl), elevated LDH (400 SU), and low glucose (40 mg/dl). The frank bloody ascites are seen in peritoneal carcinomatosis in only 10% cases, and blood-tinged (RBC >10,000 mm) ascites are seen in 8.3% of cases.[39] Furthermore, increased levels of a vascular endothelial factor (VEGF), and some other tumor markers are also found in increased amounts.[40]
Tumor Markers
To assess the effectiveness of tumor markers in diagnosing the malignancy, a study to determine the correlation of tumor markers in serum and ascites was done. Tumor markers such as carcinoembryonic antigen (CEA), CA 19-9, CA-125, CYFRA (cytokeratin fragments) were studied and reported to be highly correlated in both ascites and serum. Also, CA-125, CEA, and CYFRA are produced by normal epithelial cells and thus can be found in certain benign conditions. CYFRA was the only tumor marker found in higher quantities in malignant conditions. Still, no benefit of measuring the tumor markers in ascites than in serum was found for diagnosis.[41] However, another study later reported that the use of tumor markers along with cytology in ascites increases the diagnostic yield by 37%. The combination of 3 tumor markers (CA 19-9, CA 15-3, CEA) was reported to have a sensitivity (86%) and specificity (97%) in cases of negative cytology.[42] Regarding the significance of tumor marker measurement in ascites, different studies (using different cutoff levels of tumor markers) have rendered varying results. Hence, it is considered an unproven and unhelpful test due to its low sensitivity.
Cytology
Cytology is positive in first specimens in 83% of cases of malignant-related ascites, and yield increases to 93% and 97% if two and three samples are sent respectively.[39] It provides beneficial results except in malignant mesothelioma. The sensitivity of peritoneal cytology for detecting malignancy is 50% to 70%.[39] However, subtyping the tumor from cytology alone is difficult, and immunohistochemical (IHC) staining is required. It is essential to use the specific IHC stains for the correct tumor differentiation, grading, and providing appropriate treatment. The most commonly used stains in PC includes calretinin, cytokeratin, and BerEP4. The calretinin is a mesothelial marker and indicates the presence of both normal and malignant mesothelial cells. Calretinin and BerEP4 positive cells indicate their epithelial origin and are used for the diagnosis of EOPPC. The malignant mesothelioma stains negative for BerEP4 and positive for cytokeratin CK5/6, calretinin, and podoplanin. The DSRCT cells stains for cytokeratin, desmin, neuron-specific enolase, and WT1. The metastatic adenocarcinomas show no nuclear staining for calretinin and cytokeratins. The tumors from the upper gastrointestinal tract (GIT) are positive for CK-7 and show variable results for CDX2/CK20. In contrast, tumors from lower GIT are negative for CK-7 and positive for CDX2/CK20.[43][44] This increases the chances of detection of malignancy and its pathological subtype from ascites, especially in ambiguous cases. However, a definite role remains unclear, and therefore it is just used as an adjunct.
2. Laparoscopy
Laparoscopy is a minimally invasive procedure that provides direct visualization for a directed peritoneal lavage and tumor identification with a sensitivity of 100%. Biopsy of suspected lesions are taken during laparoscopy, and histological diagnosis is made. It is a useful tool for staging the tumor. It also prevents extensive procedures like open laparotomies by differentiating an unresectable tumor from operatable ones. It is done by calculating PCI (peritoneal carcinomatosis index), which assesses the spread of tumors in thirteen abdominal regions and each of which gets a score of 3. The total score ranges from 0-39. The higher the PCI score, the worse the prognosis. It also predicts the response of surgery in PC patients.[45][46] As compared to laparoscopy, abdominal exploration is also done by open laparotomy, which contributes to the detection of even 1-2 mm lesions.
Treatment / Management
The best therapeutic approach is adopting multimodal therapy for peritoneal cancer. A combination of surgery, chemotherapy, and targeted therapy is the mainstay of treatment. Chemotherapy includes systemic and peritoneal chemotherapy. The foundation for implementing this treatment strategy was laid by Dr. Sugarbaker. Multiple clinical trials and systematic reviews have been done over time, which emphasizes the survival benefits of multimodal therapy over the traditional palliative approach (60 months vs. 4 to 12 months).[47]
EOPPC is managed the same way as the serous ovarian carcinomas. Hysterectomy with bilateral salpingo-oophorectomy and omentectomy is done in all cases. It is followed by chemotherapy and targeted therapy with poly (ADP-ribose) polymerase (PARP) inhibitors that block DNA repair. It includes olaparib, rucaparib, niraparib, or veliparib. Platinum-based chemotherapy is favorable and used for the neo-adjuvant treatment strategy. However, many platinum-resistant tumors have proven unresponsive, and multimodal therapy is advantageous in such cases. A phase III clinical trial has indicated the superiority of intraperitoneal chemotherapy over intravenous in terms of overall survival (60 months Vs. 50 months, p=0.03).[48] Moreover, debulking surgery, defined as wide excision of the tumor with <2 cm residual nodules, also called cytoreductive surgery (CRS), is performed with chemotherapy producing optimal results in 33% to 69% patients.[1] In contrast, salvage chemotherapy is employed in tumor recurrence and incorporates doxorubicin, methotrexate, paclitaxel, and 5-fluorouracil.
In MPM, cytoreductive surgery and intraperitoneal chemotherapy (IPC) are considered the first line. In intraperitoneal chemotherapy, heated (HIPEC), or early postoperative (EPIC), data collected favors the HIPEC.[36] Drugs used are cisplatin along-with mitomycin C, melphalan, ifosfamide. Systemic chemotherapy is considered in high surgical risk and recurrent tumors. Recent advances in immunotherapy with checkpoint inhibitors have shown activity in MPM.
In DSRCT, neo-adjuvant chemotherapy is the primary approach for management. Systemic chemotherapy comprising cyclophosphamide, ifosfamide, vincristine, etoposide, doxorubicin, and mesna (P6 protocol) is done, followed by aggressive surgical excision. However, the studies on using HIPEC as an adjunct therapy are still going on. Consolidative whole abdominal radiotherapy in the pediatric population in particular, and in adults generally is essential to improve the outcome.[49]
Primary Leiomyosarcomas of the peritoneal cavity is an aggressive tumor that is managed with extensive wide margin surgeries in resectable tumors and systemic chemotherapy for metastatic disease. Pre and postoperative radiotherapy are of paramount importance.
The CRS and HIPEC have yielded tremendous evidence-based significance in this challenging metastatic disease of the peritoneum. Bypassing the hepatic metabolism and first-pass effect, intraperitoneal chemotherapy provides improved therapeutic ratio for drugs and overall better clinical outcomes.
Cytoreductive Surgery (CRS)
It is the procedure of surgically removing all the tumors from the parietal and visceral peritoneal layers, and incorporates both en-bloc resections of affected organs or tissues and peritonectomy. No tumor nodule greater than 2.5 mm is left. Electrosurgery is employed for visceral implants where surgical excision remains difficult and to limit bleeding from the visceral peritoneum. Hence, the goal is the eradication of the macroscopically visible disease. However, due to associated enhanced morbidity, patient selection for this procedure is deemed essential. The PCI (peritoneal carcinomatosis index), a measure to quantify the extent of peritoneal involvement, and the histological tumor grade are used. Good performance status of the patient is also required. The PCI score > 17 in colorectal associated PC and PCI> 12 in gastric cancer are contraindications for the surgery. The tumor involvement of crucial anatomic sites of the abdomen and multiple extra-abdominal metastatic lesions also precludes the CRS.[50] Moreover, the procedure requires technical skills and conduction of excellent hemostasis. The response of surgery is recorded by a ‘completeness of cytoreduction’ score (CCR). CCR 0 is no residual disease, CCR 1 is the minimal disease of <2.5 mm, CCR 2 is 2.5 mm-2.5 cm of the tumor, and CCR 3 is >2.5 cm of residual disease.[50] The postoperative complications are responsible for long-term morbidity and include veno-thrombotic events, operative site abscess, anastomotic leaks, fistula, and long-term intensive care stay.
Hypothermic Intraperitoneal Chemotherapy (HIPEC)
It is the process of pumping powerful chemotherapy drugs at a temperature higher than average body temperature (usually 108F/41-43C) into the peritoneal cavity for 2 hours immediately following the surgery. Hyperthermia impairs DNA repair in cells, induces apoptosis, inhibits angiogenesis, and promotes denaturation of proteins. This cytotoxic effect causes cancer cells to die at 104F while the healthy cells survive the temperature till 111F. The surgery improves the absorption ability of drugs, while the loco-regional action of chemotherapy drugs results in homogenous drug distribution and causes minimal exposure to the rest of the body. The goal of this chemotherapy peritoneal dwelling is to remove the microscopic residual disease efficiently. The agents preferred in HIPEC are mitomycin C, Oxaliplatin, cisplatin, and doxorubicin.[51] The procedure lasts for 60-100 mins.
Moreover, instead of CRS, the laparoscopic approach can also be utilized for the administration of HIPEC. Laparoscopic HIPEC was found effective in 95% of cases of refractory malignant ascites in a related systematic review of the literature.[52] It has a role in adjuvant, neoadjuvant, or palliative therapy; palliation being the major indication. The laparoscopic process produces increased intra-abdominal pressure, which can enhance the penetration ability of chemotherapy drugs. Another advantage is significantly lower morbidity and mortality, and the disadvantage is the higher recurrence rates.[53] The side effects of HIPEC are neutropenia, spontaneous bowel perforations, electrolyte imbalance, acute renal failure, and bleeding diathesis.[54]
Early Postoperative Intraperitoneal Chemotherapy (EPIC)
It is another regimen of intraperitoneal delivery of chemotherapy drugs. It is started on a postoperative day one and continued for 5-7 days. The solution containing chemotherapy drugs bathes the mesothelium for 4 to 24 hours, then is drained over 1 hour and, finally, re-administered.[55] Prior to starting the therapy, it is pertinent to look for the stable status of patients after surgery, such as normal white blood cell count and the ability to tolerate the treatment. This can delay the starting to 2nd postoperative day as well. A catheter is secured with sutures for delivery, and multiple closed suction drains are placed for drainage. The drugs introduced are cell-specific types as compared to cell cycle nonspecific drugs used in HIPEC. 5-fluorouracil, taxanes, and leucovorin are usually employed.[56]
EPIC VS. HIPEC
EPIC has the advantage over HIPEC that increased lingering and longer contact time between drugs, and the peritoneal surface is allowed, resulting in more uniform drug distribution and enhanced cytotoxic effects. The instilled solution of chemotherapy drugs covers only 30 to 40% of the peritoneal surface if allowed for a short period. Moreover, the adhesions following surgery are formed with any delay in chemotherapy induction, which renders the cancer cells entrapped in the fibrin deposits. However, the cells are still susceptible to the actions of chemotherapy drugs over five days allowed in EPIC. Further advantages are that closed abdominal technique in EPIC is easier, and drugs do not need to be heated. Therefore, the studies in mice indicated the superiority of EPIC over HIPEC in terms of overall survival.[57] However, clinical studies for comparison of the two types of intraperitoneal regimens did not regard the same. The EPIC group showed higher incidence of digestive fistulas (26% in EPIC vs 0% in HIPEC, p=0.02) and peritoneal carcinomatosis recurrence (57% vs. 26%, p=0.03). Overall survival was statistically insignificant.[58] Another study on adjunctive use of EPIC after HIPEC and CRS reported that patients who receive EPIC had increased morbidity (postoperative complications 58% in CRS+HIPEC+EPIC vs. 25% in CRS+HIPEC, p=0.048) and longer hospitalization (16 days vs. 13 days, p=0.019) with no overall change in survival.[59]
Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC)
It is a novel approach to administering intraperitoneal drugs in a minimally invasive manner. It is used as a laparoscopic palliative approach in patients in whom CRS +HIPEC is not an indication, has a considerable tumor load, or large quantities of persistent ascites. The goal is to alleviate symptoms and improve quality of life (QoL). The procedure involves connecting a device called Capnopen with an injector and inserting it into the peritoneum through a trocar. There is a nebulizer technology that pressurizes the chemotherapy drugs into aerosols. The process takes 1 hour, and drugs like cisplatin and doxorubicin are used. The drugs are removed after 30 mins by a closed suction system. Through PIPAC, drug delivery is done in a repeated, safe way without adverse systemic effects. This pressurized delivery results in the requirement of smaller doses with resulting higher drug concentration, deeper penetration, and an effective uniform drug distribution.[60] The chemical bowel perforations are decreased as compared to HIPEC. The renal and hepatic toxicity post-PIPAC is minimal to zero.[61] However, it cannot be performed in cases of biliary or small bowel obstructions and extra-abdominal metastasis.
The studies on the use of PIPAC in PC from intestinal, appendiceal, gastric, and ovarian cancers have emphasized its safety, better tolerability, and control on ascites production.[62][63][64] The median survival after PIPAC is 15.7 months.[65] However, the disadvantages are that the aerosols cannot access some anatomic locations in the peritoneal cavity, and the adhesions secondary to surgery create obstacles to aerosol diffusion. Thus, it is not a good option in patients in the early course of the disease or recurrence after CRS.[66] Moreover, tumor response following PIPAC alone is insufficient, and the addition of systemic chemotherapy can improve clinical response and QoL. This rational approach is referred to as bidirectional treatment and results in PCI improvement from 50% to 88%.[67] Hence, more clinical trials are required to test for its efficacy and usage.
Differential Diagnosis
- Peritoneal lymphomatosis
- Peritoneal tuberculosis
- Granulomatous peritonitis from histoplasmosis
- Peritoneal melanosis
- Gliomatosis peritonei
Staging
Primary PC are always classed as stage III and IV. Thus, high-grade adenocarcinomas that develop from the mesothelium of the peritoneal cavity are the extrauterine adenocarcinomas of Mullerian epithelial origin and are staged like ovarian carcinomas.[68]
STAGE III is the confinement of the tumor within the peritoneal cavity and has further subdivisions as follows:
IIIA: Cancer is found in pelvic organs and lymph nodes within the abdominal cavity.
IIIB: Cancer has spread to the peritoneum outside the pelvis, and the tumor size in the peritoneum is 2 cm or less. Also, lymph nodes outside the peritoneum will be involved.
IIIC: Cancer involves the peritoneum outside the pelvis, and the tumor size in the peritoneum is greater than 2 cm. It also has spread to lymph nodes outside the peritoneum.
In stage IV, cancer has metastasized to other organs of the body. It is further subdivided as:
IVA: Malignant pleural effusions develop
IVB: Cancer has spread to organs and tissues outside the peritoneal cavity, such as the liver, lungs, or groin lymph nodes
Secondary PC was described by Gilly et al. and is classed as follows:[69]
- 0: no macroscopic disease
- I: Malignant lesion of size < 5mm localized to one part of the abdomen.
- II: Lesion is < 5mm but diffuses to the whole abdomen.
- III: Malignant granulations are >5mm but <2cm
- IV: Large malignant cakes > 2cm
Prognosis
PC is considered a terminal illness with a poor prognosis. Whether cancer originates in the peritoneum or spreads from somewhere else, it is advanced and stage IV. The primary peritoneal cancer has a survival rate varying from 11-17 months.[70] In secondary peritoneal cancer, the median survival is six months in accordance with the stage of cancer (5-10 months for stages 0, I, and II, and 2-3.9 months for stage III-IV). It is almost similar for both synchronous and metachronous PC. Survival rates of PC also differ according to the location of the primary tumor; pancreatic origin has worst (2.9 months), followed by gastric (6.5months) and colorectal origin (6.9 months).[67] The concomitant presence of ascites and hepatic metastasis are negative survival factors.
The factors affecting EOPPC in the univariate analysis include patient age, stage, performance status, and residual tumor size after CRS, and in multivariate analysis performance status (p<0.001) and residual tumor size (p=0.03) are significant.[71]
In MPM, histological type holds prognostic significance, with the epithelioid subtype having the best prognosis. In a study, median survival in epithelioid MPM was 55 months compared to 13 months in the biphasic subtype. Moreover, sarcomatoid features (p=0.0006), depth of invasion (p=0.02), CCR 2-3 (p=0.02), and inflammatory stroma (p=0.04) were other factors associated with worse prognosis.[72]
In DSRCT, the prognosis is dismal, with a 5-year survival of only 15% to 30%. The extra-abdominal presence of tumors is associated with worse prognosis, while surgical resection in loco-regional disease and radiotherapy in metastatic disease offers better survival.[73]
The stage and size of leiomyosarcoma are the two factors influencing its overall bad prognosis (64% at five years).[10]
Prognostic factors associated with secondary PC from GIT tumors include tumor histopathology, stage of the tumor, PCI score, and CCR score.[74] A study done by Vaira, M. et al. showed that CCR is strictly related to overall survival, and PCI has no statistically significant effect on overall survival.[75] While in PC from ovarian cancer; low grade, small volume tumors, adjuvant chemotherapy in treatment, and complete CCR (p<0.05) were associated with favorable outcomes, and PCI is the best indicator for survival (p=0.0253).[76]
Enhancing Healthcare Team Outcomes
Primary peritoneal cancer is a rare malignancy, and dissemination from the primary organ site represents the late stage of cancer. Both manifest a poor prognosis. The bewildering presentation, intricate diagnostic methods, composite management, adverse treatment effects, and despair related to the news of this stage IV are the hurdles associated with it. It demands a competitive and cooperative team consisting of nurses, radiologists, surgeons, oncologists, and psychiatrists to manage the disease physically and psychologically. The continuous uplifting of the spirits of patients during the advanced surgery and perilous chemotherapy is critical. The advancement in technology and the development of HIPEC has increased the survival rate and improved the quality of life.
Further, new non-invasive detection methods should be developed for peritoneal cancer such as liquid biopsy (serum and ascitic fluid) containing biomarkers consisting of ‘exosomes,’ that protect cancer cells from degradation.[77] This is essential because imaging techniques in variable diseases involving peritoneum are similar. Moreover, standardization of CRS + HIPEC is the need for time together with discovering new ‘targeted molecular therapy and immunotherapy.’ This revolutionary management will help in decreasing morbidity and mortality associated with the fatal disease.
(Click Image to Enlarge)
(Click Image to Enlarge)