Permethrin
- Article Author:
- Japbani Nanda
- Article Editor:
- Andrew Juergens
- Updated:
- 5/30/2020 8:25:27 PM
- For CME on this topic:
- Permethrin CME
- PubMed Link:
- Permethrin
Indications
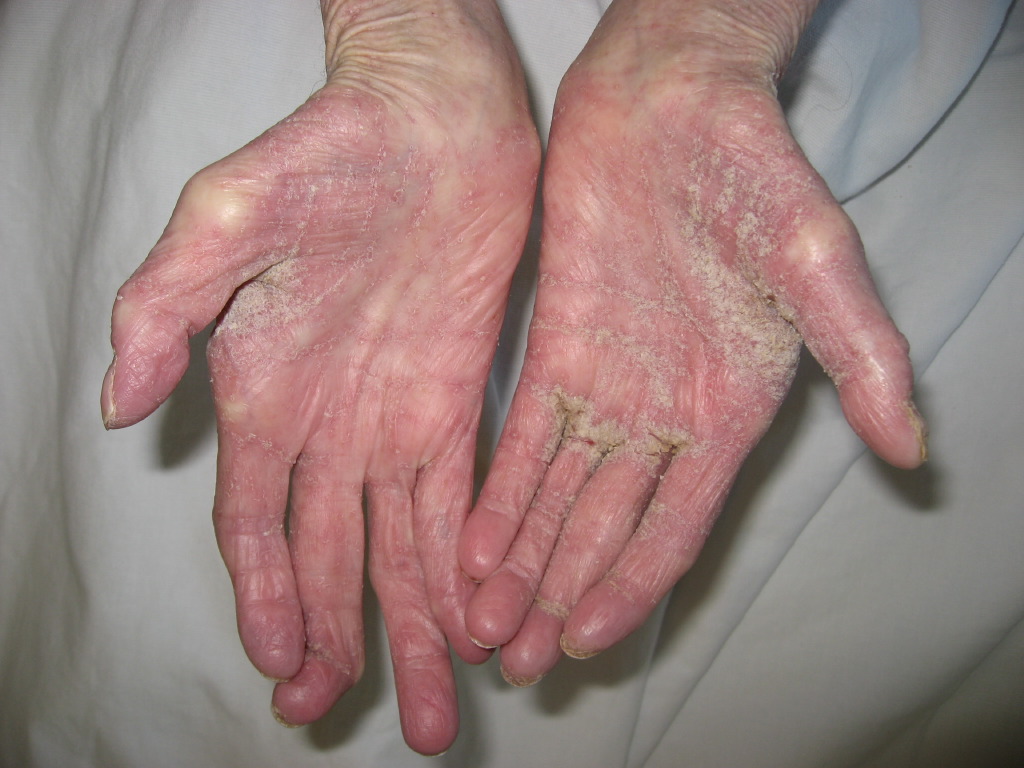
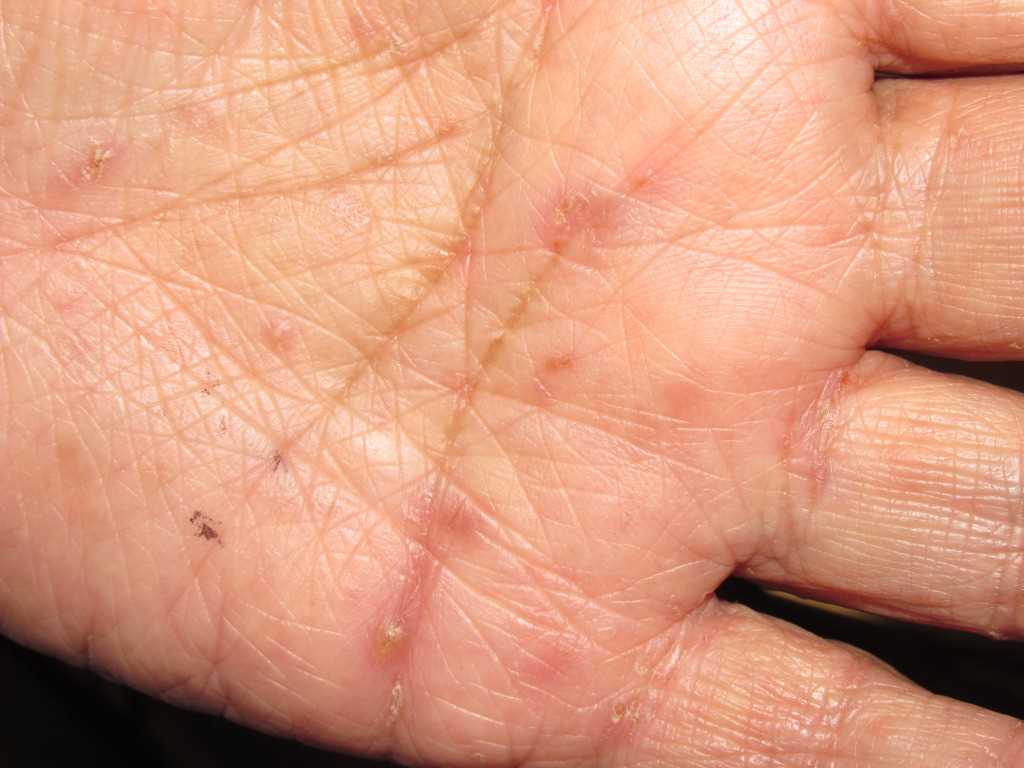
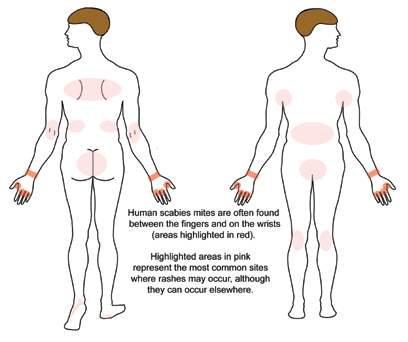
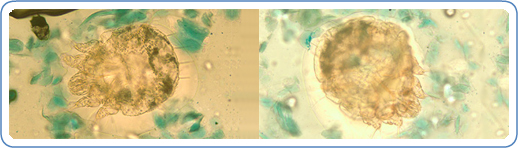
The United States Food and Drug Administration (FDA) has approved the use of permethrin, a synthetic pyrethroid, for the treatment of scabies and pediculosis capitis.[1] A patient with classic scabies often presents with generalized pruritus that is exacerbated overnight with the presence of inflammatory papules primarily localized to the area between the fingers, flexural sites, genitalia, breasts, and buttocks with or without burrows.[2] The clinical diagnosis of pediculosis capitis relies upon the detection of living lice.[3]
Several studies have assessed the effectiveness and safety of permethrin when compared with other treatments for scabies. A review of clinical trials resulted in low-certainty evidence demonstrating that after two weeks of administration, 5% permethrin cream and 200 micrograms/kilogram of oral ivermectin demonstrated similar efficacy for the treatment of scabies, but clearance after the first week may be inferior when using oral ivermectin. There was moderate-certainty evidence to conclude that the proportion of patients with one or more drug-related adverse events was similar in those treated with permethrin cream or oral ivermectin.[4] A recent network meta-analysis evaluating randomized controlled trials found that treatment with combined permethrin and oral ivermectin for scabies was associated with the highest rate of cure; however, combination treatment was not significantly better than permethrin alone, and only one randomized controlled trial included this combination treatment. This analysis also found that permethrin was less often associated with persistent itching than other included treatments, like crotamiton and lindane, but was outperformed by topical ivermectin in this category. The risk of adverse events was lowest with synergized pyrethrins, but permethrin scored better than several other treatments, such as lindane and topical ivermectin. The treatment of choice for a particular patient should be guided by several factors, which include efficacy, safety profile, and the ability to administer the medication with ease properly.[5]
Classical scabies can have treatment with 5% permethrin cream, 200 micrograms/kilogram of oral ivermectin, or 10%-25% benzyl benzoate lotion per European guidelines.[6] In the United States, 5% topical permethrin cream is a first-line treatment option for scabies. For the treatment of crusted scabies, patients may benefit from combined treatment with a topical medication, such as 5% permethrin cream, and 200 micrograms/kilogram of oral ivermectin.[2] However, the best treatment for crusted scabies remains undetermined due to a lack of sufficient research in this area.[7] In endemic regions and during a scabies epidemic, large numbers of people can more easily receive treatment with 200 micrograms/kilogram of oral ivermectin than with topical medications.[6] Also, close contacts and sexual partners during the prior two months should receive treatment.[2][8] It is also essential to maintain personal hygiene and wash items, such as clothes and bedsheets, at high temperatures.[6] Items can also be sealed within a bag for a minimum of two to three days as an alternative.[2] Some patients may continue to experience pruritus for a maximum of four weeks after proper treatment.[2]
A common first-line treatment option for pediculosis capitis is 1% permethrin lotion, which is available over the counter in many countries.[9] A systematic review of trials that evaluated medications for the treatment of pediculosis capitis suggested that permethrin was efficacious.[10] However, since then, permethrin has been associated with wide-spread resistance, and newer options that physically target lice, such as dimethicone, have been studied.[11][12] A recent trial found that treatment of pediculosis capitis with dimethicone was associated with significantly higher cure rates than treatment with 1% permethrin lotion.[13] The best treatment option for a particular patient must consider local resistance.[3]
Concerning pediculosis, pediculicide product labels often only address their use for the treatment of pediculosis capitis. Treatment of pediculosis corporis relies upon good personal hygiene and appropriate washing of clothes, but a topical pediculicide could aid in treatment, as well.[14] A 1% permethrin cream has been recommended for the treatment of pediculosis pubis as part of a broader regimen that includes cleansing of clothes and linens, sexual abstinence during treatment, and treatment of sexual partners from the prior month.[7] Meanwhile, European guidelines recommend treatment of sexual partners from the prior three months.[8]
Permethrin has not been shown to cause human risk and has, therefore, was in the former FDA pregnancy category B.[2] Permethrin is the preferred medication for pediculosis pubis and scabies during pregnancy and while breastfeeding.[7] However, if a breastfeeding woman with scabies applies permethrin directly to affected breasts, then they should bottle-feed the baby until they have rinsed the cream off completely.[2] Permethrin can also be used in infants starting at the age of 2 months.[15]
Mechanism of Action
Administration
For classical scabies, 5% permethrin cream is applied topically to cool, dry skin from the patient’s head to the patient’s toes and under the fingernails.[6] The cream is washed off after 8 to 14 hours and is often reapplied in the same way one week later.[2] As per European guidelines for crusted scabies, 5% permethrin cream can be applied topically every day for one week, followed by application twice a week until resolution, along with 200 micrograms/kilogram of oral ivermectin administered at a specified dosing interval over several days.[6] Patients should avoid contact with mucosal sites when applying topical permethrin.[2]
For the treatment of pediculosis capitis, apply 1% permethrin to dampened hair and washed off after ten minutes with repeat application one week later.[17] However, other reapplication timeframes have been proposed, such as reapplication 7 and 13 to 15 days after initial treatment.[18] For the treatment of pediculosis pubis, 1% topical permethrin cream is rinsed off 10 minutes after application.[7]
Adverse Effects
Adverse effects reported during the treatment of scabies with permethrin include increased or new onset of pruritus, burning, or a stinging sensation. These dermatologic adverse events were mild in severity and temporary.[19] There have also been reports of stinging during the application of 1% permethrin for pediculosis capitis.[20] Additionally, there are also reports of rash with the use of permethrin for pediculosis capitis.[21] Localized temporary paresthesia, cutaneous irritation, and allergic contact dermatitis are also possible, yet rare, adverse effects associated with the use of permethrin.[22] Although rare, a case report exists of a patient with muscle dystonia that was probably related to the use of 5% topical permethrin.[23]
Contraindications
Permethrin has not been approved by the FDA for use in infants before the age of 2 months, and limited studies have taken place to assess the use of this medication in these patients.[15] However, some recent research suggests that 5% permethrin cream can be safely used for the treatment of scabies in this infant population.[24] Also, care is necessary to avoid inappropriate administration because some patients may be allergic to the medication’s formulation.[25]
Monitoring
The use of topical permethrin is associated with limited dermal or systemic absorption.[26] Less than two percent of applied permethrin is absorbed through human skin.[1] Permethrin quickly undergoes esterase hydrolysis and detoxification within the body.[1] The liver carries many of the carboxylesterases that hydrolyze permethrin.[27] The liver also has cytochrome P450 enzymes that oxidize permethrin into metabolites.[28] During the 72 hours after topical application, permethrin’s inactive metabolites undergo nearly complete excretion into the urine.[1] In humans, the postulated lethal oral dose of permethrin is 1 to 2 grams/kilogram of human body weight.[26]
As per European guidelines, patients with scabies should return for follow-up two weeks after treatment has ended to assess for resolution.[6] If symptoms of pediculosis pubis remain, patients should return for follow-up one week later to assess for remaining lice or eggs.[7]
Toxicity
Dermal absorption of topical permethrin is limited, and the drug has a minimal systemic presence in humans with no risk of accumulation over time.[26] The risk of toxicity with the use of 5% permethrin cream was estimated to be forty to four hundred times smaller than that associated with the use of 1% lindane lotion.[29] Permethrin is known to possess limited toxicity in mammals, and the topical formulation contains more of permethrin’s trans-isomer because the cis isomer is associated with more toxicity.[1]
Although reports of toxicity exist when using permethrin as an insecticide, there are only a few adverse events associated with its topical use.[23] Signs of toxicity and metabolic acidosis were reported following the excessive application of 5% topical permethrin when treating a 20-month-old patient with scabies.[30] Also, two siblings who had exposure to permethrin, a type I pyrethroid pesticide, experienced neurotoxic effects.[31] Clinicians pursued supportive treatment because there is no antidote for permethrin toxicity.[31]
Enhancing Healthcare Team Outcomes
The coordination of an interprofessional team of health care providers is critical to ensure optimal health care outcomes for patients who receive therapy with topical permethrin for scabies or pediculosis. Patients with scabies or pediculosis may present to a variety of different health care professionals in various fields, such as primary care, urgent care, emergency medicine, pediatrics, family medicine, internal medicine, or dermatology. School nurses and health professionals may identify a child with pediculosis capitis and facilitate communication with the child’s caregivers so that timely treatment can start.[9]
Treatment outcomes and safety depend on the proper use of topical permethrin.[2] Therefore, patients and caregivers should receive written instructions for the application of topical permethrin in addition to verbal explanations. Such instructions are also essential to avoid inadvertent misuse or overuse of the medication. Nurses, nurse practitioners, physicians, and physician assistants can help ensure that patients and caregivers understand medication administration instructions and can monitor for adequate treatment response. Pharmacists may also clarify these instructions if questions arise, as well as verifying dose and ensuring ther eare no drug interactions in play.
In areas with local resistance to permethrin, other treatment options should be options.[3] Pharmacists can work with physicians and other health professionals to find an appropriate medication for each patient. Also, patients with pediculosis pubis should be assessed for other sexually transmitted infections and may need a referral to an appropriate specialist.[7] There must be coordinated care between many healthcare team members because close contacts and sexual partners of patients with scabies and pediculosis pubis should have treatment to prevent re-infestation.[2][8][7] [Level 5] Coordination of health care efforts allows patients to benefit from the care of an organized interprofessional team of health professionals.
(Click Image to Enlarge)
(Click Image to Enlarge)