Pilocytic Astrocytoma
- Article Author:
- James Knight
- Article Editor:
- Orlando De Jesus
- Updated:
- 7/15/2020 10:50:27 PM
- For CME on this topic:
- Pilocytic Astrocytoma CME
- PubMed Link:
- Pilocytic Astrocytoma
Introduction
Pilocytic astrocytoma (PA), previously known as cystic cerebellar astrocytoma or juvenile pilocytic astrocytoma, was first described in 1931 by Harvey Cushing, based on a case series of cerebellar astrocytomas.[1] They are low-grade, and usually well-circumscribed tumors, which tend to occur in young patients. By the World Health Organization (WHO) classification of central nervous system tumors, they are considered grade I gliomas and have a good prognosis.[2]
PA can occur in the optic pathway, hypothalamus, and brainstem. They can also occur in the cerebral hemispheres, although this tends to be the case in young adults. Presentation and treatment vary for PA in other locations, and this article will discuss the PA of the cerebellum only. Glial cells include astrocytes, oligodendrocytes, ependymal cells, and microglia. Astrocytic tumors arise from astrocytes and are the most common tumor of glial origin. The WHO 2016 categorized these tumors as either "diffuse gliomas" or "other astrocytic tumors." Diffuse gliomas include grade II and III diffuse astrocytomas, grade IV glioblastoma, and diffuse gliomas of childhood. The "other astrocytic tumors" group include PA, pleomorphic xanthoastrocytoma, subependymal giant cell astrocytoma, and anaplastic pleomorphic xanthoastrocytoma.[2]
The above-described grading system is histological. It is worth noting that the new 2016 WHO classification gives more importance to genetic and molecular markers for categorizing gliomas.
Etiology
There is a strong association between neurofibromatosis type 1 (NF1) and PA, with up to 20% of patients with NF1 developing a PA most commonly in the optic pathway.[3][4] However, most PAs are thought to be sporadic mutations rather than inherited. BRAF gene alterations and MAPK signaling pathway alterations have been found in the majority of PAs.[5][6][7] BRAF is an intracellular serine/threonine kinase involved in the activation of the mitogen-activated kinase (MAPK) pathway.[8] BRAF is a proto-oncogene, mutations of which have been found to cause human cancers.[9][10]
Epidemiology
Brain tumors are the most common solid cancer in childhood.[11] PA is the most common childhood brain tumor, with an incidence of 0.8 per 100,000 people.[12][13] PA most often presents in the second decade of life, with 75% occurring before the age of 20 years.[14] PA accounts for 15% of all brain tumors in children and comprises 27 to 40% of all pediatric posterior fossa tumors.[15][16] They can occur in adults, though typically in young adults.[17] PA in adults comprises 5% of all primary brain tumors, located most commonly at the temporal and parietal lobes.[18]
Pathophysiology
PA tends to occur close to the midline, although, in an adult, it can be more lateral within the cerebellum. PAs can occur anywhere within the neuroaxis:[16]
Histopathology
PA is a WHO grade I tumor. There are case series of anaplastic PA, with uncertain behavior.[20]
Microscopic features
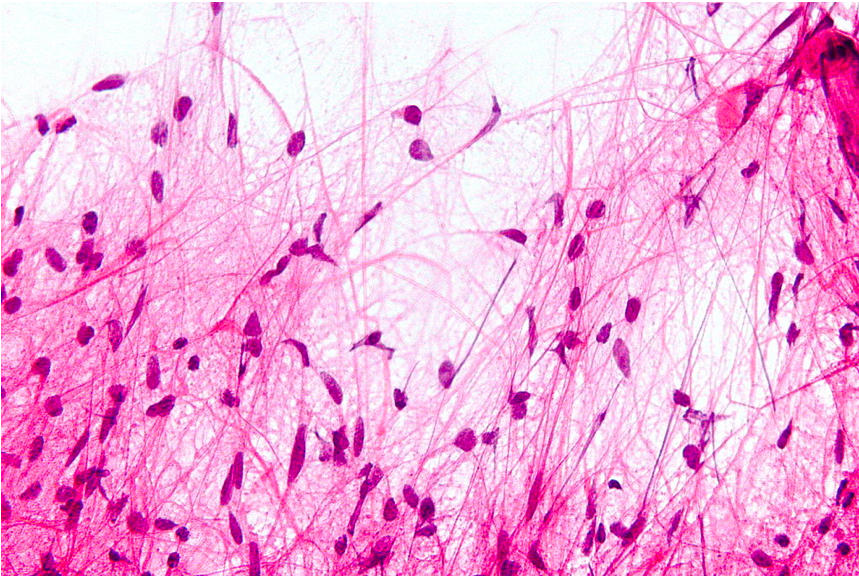
PA gets its name from the microscopic appearance of cells with long, thin bipolar processes that resemble hairs (hence pilocytic).[5] There are often Rosenthal fibers, which are elongated eosinophilic bundles that are found on hematoxylin and eosin staining. PAs have low to moderate cellularity. Multinucleated giant cells with peripheral nuclei can be seen. Tumors that have been present for a more extended period may have hemosiderin-laden macrophages and calcifications.[21] Areas of necrosis may be seen.
Though they are radiologically described as well-circumscribed, nearly two-thirds infiltrate the surrounding brain parenchyma.[22][23] Histological architecture shows that PA frequently breaks through the pia and breach into the subarachnoid space. They can infiltrate the perivascular spaces.
Microscopically, it can be challenging to differentiate between PA and low-grade diffuse astrocytoma.[2] For this reason, the pathology team needs to have demographic and radiographic information available to guide diagnosis. Small biopsy samples also compound this diagnostic issue.
Molecular features
The most common genetic abnormality found in 70% to 75% of PAs is BRAF gene alterations.[6] BRAF alterations are more common in pediatric PA than in adult PA.[24] PAs have alterations in the MAPK signaling pathway in more than 80% of the cases.[5][7][25] KIAA1549-BRAF fusion is the most common identified mutation in PA.[8][24] Immunohistochemistry shows positivity for glial fibrillary acidic protein, S100 protein, and oligodendrocyte transcription factor. Importantly, as with other pediatric low-grade gliomas, they are negative for isocitrate dehydrogenase (IDH) and tumor protein p53 (TP53) mutations.[2][15]
History and Physical
PA can present with symptoms secondary to the posterior fossa mass effect. This may include obstructive hydrocephalus, with resultant headache, nausea and vomiting, and papilledema. If hydrocephalus occurs before the fusion of the cranial sutures (<18-months-of-age), then an increase in head circumference will likely occur.[26]
Lesions of the cerebellar hemisphere result in peripheral ataxia, dysmetria, intention tremor, nystagmus, and dysarthria. In contrast, lesions of the vermis cause a broad-based gate, truncal ataxia, and titubation. Posterior fossa lesions can also cause cranial nerve palsies. Diplopia may occur due to abducens palsy from the stretching of the nerve. They may also have blurred vision due to papilledema. Seizures are rare with posterior fossa lesions.[26]
Evaluation
The investigation of choice is brain magnetic resonance imaging (MRI) with contrast. This also avoids radiation exposure. However, if the child is neurologically unstable, an urgent head computed tomographic (CT) scan with contrast is indicated. With malignant pediatric posterior fossa lesions, the whole neuroaxis must be imaged to assess for metastasis. Spinal seeding or leptomeningeal dissemination is extremely rare in PA; therefore, it is only recommended in suspected or uncertain cases.[27][28][29][30]
PA can have multiple radiological appearances. In 66%, there is a significant cystic component with an avidly enhancing mural nodule, while in 46%, the cyst wall also enhances.[31] In up to 17% of the cases, the tumor is solid with minimal or no cystic component.[22] Up to 20% may show calcification. PA is periventricular in 82% of cases.[22] The cyst content is proteinaceous with an average measurement of 4 Hounsfield units, which is denser than the cerebrospinal fluid (CSF).[32] On MRI, the mural nodule is hyperintense on the T2 image and iso or hypointense on the T1 image. The cyst content shows a high signal on the T2 image, similar to the CSF.
Treatment / Management
Management of the tumor
The mainstay of treatment is surgical excision with the aim of complete resection margins while achieving minimal neurological injury. Complete resection is considered curative for the disease. However, the involvement of the brainstem or cranial nerves may prevent complete resection. Resection of only the nodule and not the cyst wall is recommended.[31] However, tumors with a thick cyst wall, can be considered part of the nodule and thus removed.[31]
Conventional radiotherapy is not required; instead, follow up with serial imaging is more appropriate. Radiotherapy, particularly in this region of the brain, carries significant sequelae.[33] If there is a recurrence, further surgical resection is typically adopted. Radiotherapy may be appropriate if surgically unresectable or if malignant histology is present.[34] Stereotactic radiosurgery offers excellent results for residual and recurrent tumors.[35]
Management of hydrocephalus
Approaches for neurologically well patients
- CSF diversion at the time of surgery, followed by immediate tumor resection
- Surgical resection without CSF diversion. If hydrocephalus persists postoperatively, then perform CSF diversion
- CSF diversion around two weeks before definitive surgery
If the patient is neurologically unstable due to hydrocephalus or brainstem compression, then urgent intervention is warranted. If the presentation is with hydrocephalus, some authors advocate initial CSF diversion before definitive surgery using an external ventricular drain (EVD), endoscopic third ventriculostomy (ETV), or ventricular-peritoneal shunt (VPS). Some centers will place an EVD or perform an ETV at the time of surgery, followed by immediate definitive tumor resection. However, some advocate that CSF diversion should be around two weeks before resection.[36]
Disapproval for this approach is that placing a VPS generally commits patients to lifelong shunting and complications. Also, tumor seeding may occur into the peritoneum, although this is rare and can be mitigated by the placement of a tumor filter on the shunt system.[37] Surgical factors to consider with CSF diversion include possible upwards transtentorial herniation and CSF infection due to VPS or EVD contamination. Some criticize that this method causes unnecessary delays to definitive treatment.
Differential Diagnosis
Most common pediatric posterior fossa tumors:[26][38]
- Medulloblastoma (typically midline, roof of fourth, vermian, <10% have calcification)
- Diffuse pontine glioma (usually multiple cranial nerve palsies)
- Ependymoma (usually arise in the floor of the fourth ventricle, calcification common)
Other pediatric posterior fossa lesions:
- Hemangioblastoma
- Atypical teratoid/rhabdoid tumor
- Cerebellar abscess
- Choroid plexus papilloma
- Metastasis: neuroblastoma, rhabdomyosarcoma, Wilm’s tumor
Adults, posterior fossa tumors:
Prognosis
PA is a slow-growing tumor that is often curative on resection. The best prognostic factor is total resection. The survival rate at 10-years is approximately 95% if completely resected.[13][41] Recurrence is rare if complete resection is achieved. Those that do recur tend to do so within a few years.[42] Collins' law states that "the period of risk for tumor recurrence is the age of the child at diagnosis plus nine months".[43] Using this concept, PA can be considered cured if it does not reoccur within that time. However, there are documented cases of late recurrence.
If there is incomplete resection, recurrence will occur with the progression of symptoms. Risk factors for recurrence have been identified, including solid tumor, exophytic component, and tumor invasion.[44] Patients younger than one year have the worst prognosis.[13] For PA in adults, the survival at 5-years is 85%, and progression-free is 70%.[45] Tumor recurrence in adult cases is approximately 20%.[45]
For completely removed tumors, a maximum of three-year surveillance imaging is recommended due to the minimal risk of recurrence in pediatric PA.[46]
Complications
Patients with posterior fossa tumors may develop hydrocephalus requiring a VP-shunt. If so, they will likely be shunt-dependant for life.[36][47] Though recurrence is usually amenable to further resection, some PAs show malignant degeneration, though this is rare. Most of the cases with malignant degeneration seem to follow radiotherapy.[48][49]
Deterrence and Patient Education
Pearls and Other Issues
- PA is the most common pediatric brain tumor
- Classified by the WHO as low-grade, grade I
- MRI: Typically cystic with avidly enhancing mural nodule; although can be completely solid
- Microscopy: long, bipolar cellular processes that look "hair-like"
- Excellent prognosis with complete surgical resection; radiotherapy and chemotherapy not routinely used
- 95% 10-year survival with surgery alone if complete resection
- Strong association with NF1, 5% to 20% of patients with NF1 develop PA mostly in the visual pathway
- Presentation typically with cerebellar signs and features of hydrocephalus
- Patients may require CSF diversion
Enhancing Healthcare Team Outcomes
PA is the most common pediatric brain tumor and requires urgent assessment and management. These patients will initially present to pediatricians, emergency clinicians, or general practitioners. Therefore, these clinicians must recognize signs and symptoms of masses affecting the cerebellum or brainstem, including the clinical presentation of hydrocephalus.
An interprofessional team will be involved in decision making, which will include neurosurgeons, oncologists, radiologists, neurologists, and primary clinicians. Perioperatively, the team will consist of: neurosurgeons, anesthetists, intensivists, primary clinicians, and nursing teams. During the postoperative and rehabilitation period, physiotherapists will help the patient with activities of daily living.