Plasmodium Falciparum Malaria
- Article Author:
- Lara Zekar
- Article Editor:
- Tariq Sharman
- Updated:
- 10/1/2020 11:28:45 PM
- For CME on this topic:
- Plasmodium Falciparum Malaria CME
- PubMed Link:
- Plasmodium Falciparum Malaria
Introduction
Malaria is a mosquito-borne disease caused by five protozoa: Plasmodium falciparum, P. vivax, P. malariae, P. ovale, and most recently implicated P.knowlesi. Infection with P. falciparum is being accounted for more than 90% of the world’s malaria mortality and therefore remains an important threat to public health on a global scale.[1][2] The World Health Organization (WHO) World Malaria report 2019 estimates 228 million cases of malaria worldwide, causing 405 000 deaths in the year 2018, many under the age of 5. Malaria is endemic in more than 90 countries, affecting approximately 40% of the world’s population.[2] There is a significant number of cases of imported malaria and local transmission following importation occurring in non-malarial countries, including North America and Europe.[3] Malaria is associated with travelers to the endemic areas, and increasing numbers of imported malaria necessitate an understanding of frequently non-specific symptoms, difficulties related to the malarial diagnosis, and treatment possibilities.[2]
Etiology
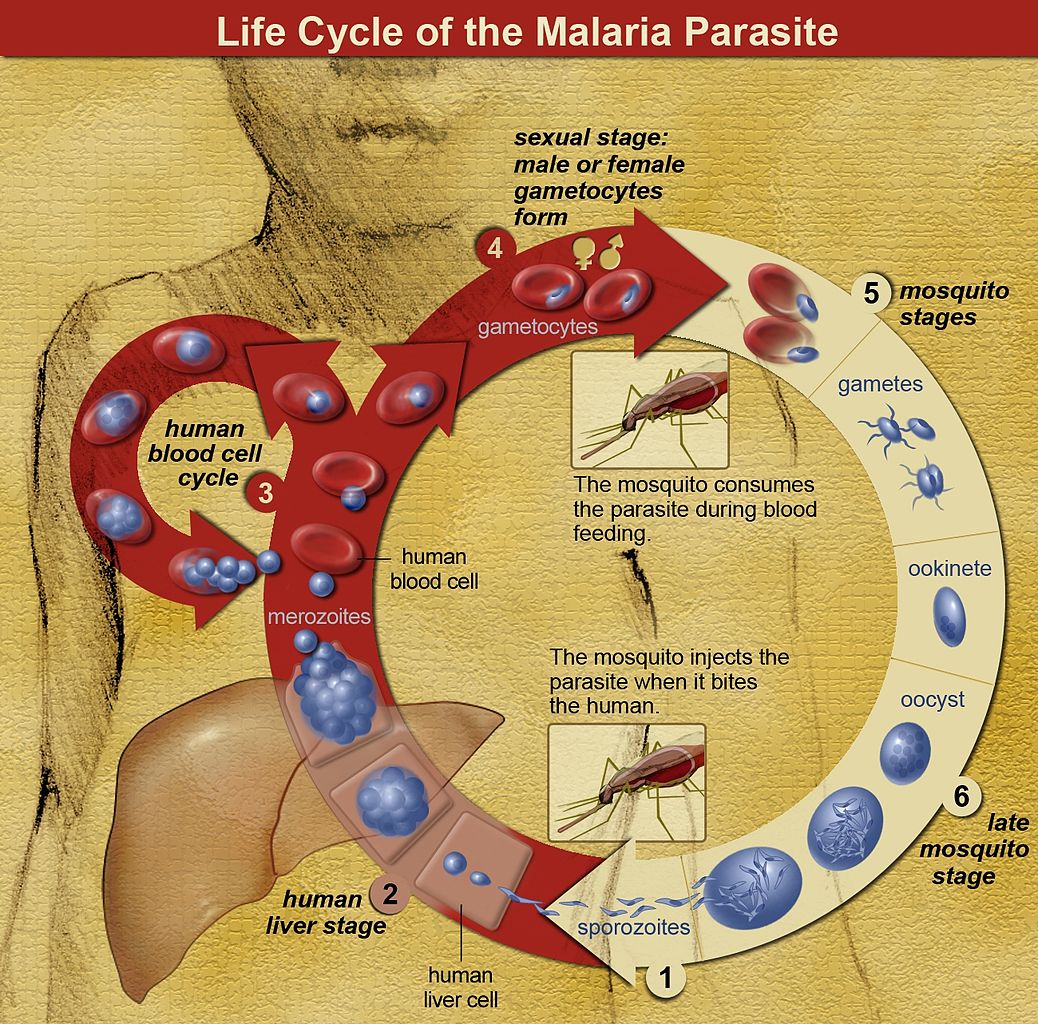
Five species of genus Plasmodium are known to cause malaria in humans. The vector for Plasmodium spp. is a female Anopheles mosquito that inoculates sporozoites contained in her salivary glands into the puncture wound when feeding.[3] Sporozoites enter peripheral bloodstream and are uptaken by hepatocytes, where they undergo an asexual pre-erythrocytic liver-stage as liver schizonts lasting up to 2 weeks before the onset of the blood stage.[3][4] As they replicate within hepatocytes, they form motile merozoites that are subsequently released into the bloodstream, where they invade red blood cells (RBC). The process continues through serial cycles of asexual replication of merozoites that go through ring, trophozoite, and schizont stages before forming and releasing new invasive daughter merozoites that consequently infect new RBC, therefore causing a rise in parasite numbers.[3][5] P. falciparum produces high levels of blood-stage parasites and is known to modify the surface of the infected RBC, creating an adhesive phenotype, e.g. (sticky cell) causing RBC sequestration inside small and middle-sized vessels, removing the parasite from the circulation for nearly half of the asexual cycle.[6] Sequestration leads to splenic parasite clearance avoidance, host cell endothelial damage, and microvascular obstruction.[5][6] A small fraction of intra-erythrocytic parasites switch to sexual development, producing morphologically distinct male and female gametocytes that reach the host's dermis and are ingested by a mosquito, rendering it infectious to humans.[3][4][5] After ingestion by a female Anopheles mosquito, the male micro-gametocytes go through a process of ex-flagellation in the mosquito's midgut, fusing with female macro-gametes to form a zygote. The zygote then reaches the stage of ookinete that migrates through a thin wall, matures into oocyst, producing and upon rupturing, releasing numerous sporozoites that are dispersed throughout mosquitos body, including salivary glands, therefore completing the lifecycle. Gametocytes are hence of vital importance to the transmission cycle of malaria.[2][4][7] The clinical symptoms are, however, predominantly a result of the asexual stages of parasite replication in human blood.[5]
Epidemiology
Worldwide
WHO World Malaria Report 2019 states that an estimated 228 million cases of malaria occurred worldwide in 2018, and reports steadily decreasing the number of cases since 2010. In 2018, nineteen sub-Saharan African countries and India carried approximately 85% of the global malaria burden. The most prevalent and pathogenic malaria parasite, most commonly associated with severe illness and death, especially in the WHO African region, accounting for 99.7% malaria cases, is P. falciparum.[8] P. falciparum is also highly prevalent in South-East Asia, Eastern Mediterranean, and Western Pacific regions. The most vulnerable groups affected by malaria in high-transmission areas are children younger than 5 years old, their deaths accounting for 67% of global malaria deaths, and primigravidae. In low transmission areas, all ages are at risk due to low immunity.[2]
The United States
Most malaria cases diagnosed in the United States of America are imported from endemic countries.[9] The risk of infection depends on the length of exposure and the intensity of malaria transmission in the geographical region.[10] During 2015 CDC received 1517 reports of confirmed malaria in the United States of America, one of the cases was classified as congenital, 1485 were imported from endemic countries, and 31 reports had an incomplete travel history. The 12.1% decrease of imported malaria in 2015 correlates with the decrease in the number of cases imported from West Africa, possibly due to altered travel because of the Ebola epidemic. However, the overall trend shows that, on average additional 29 cases of malaria are reported every year since 1973, coinciding with an increasing number of international travel. Among the cases with species determination, the majority were P. falciparum, accounting for 86.6% cases from Africa, 70.9% from Central America, 20.8% from South America, and 4.8% from Asia.[8]
Based on present predictions for climate change, researchers predict an increase in the geographical distribution of malaria and an increasingly suitable climate for malaria transmission in tropical regions.[11] However, several other determinates factor in the epidemiology of malaria other than global warming: such as politics, economic development, urbanization, and population growth, migration changes, etc.[3][11] Additionally, there is an impending threat of artemisinin- and multidrug-resistant P. falciparum, particularly prevalent in Greater Mekong Subregion (GMS), causing high failure rates of artemisinin-combination therapies.[12][3]
General Epidemiology and Risk Groups
Severe malaria occurs in patients with no or little effective immunity. In parts of the world with the stable and intense transmission of P. falciparum, severe malaria is mostly a disease of the pediatric population younger than 5 years, as specific acquired immunity develops with age (due to repeated infections), providing increased, although incomplete, protection in older children and adults. Severe malaria can, however, occur at any age in areas with low or/and unstable transmission rates and individuals with no-immunity (e.g., travelers to the endemic areas).[13] Susceptibility to malarial infections increases during pregnancy.[14] Pregnant women in the second trimester are at the greatest risk of infection, although the risk is somewhat affected by age and gravidity, with young primigravidae being at the highest risk at high transmission areas.[15] Women living in areas with unstable and low malaria transmission rates are infected infrequently and therefore lack the immunity, which often causes a rapid progression to severe malaria and death.[14]
Pathophysiology
The rupture of the first liver schizont and the release of motile merozoites into peripheral circulation to invade red blood cells marks the start of a possible symptomatic infection. The first rupture and invasion are usually silent in most infected patients, but as the asexual cycle repeats itself in the next 24 to 48 hours, parasitemia rises, and immune response increases accordingly. It is usually associated with an increase of TNF alpha and other inflammatory markers in the cascade, including interleukin 10 (IL-10) and interferon-gamma (IFN-gamma).[6] Higher parasitemias are generally associated with a more severe clinical picture, but the relationship is very variable.[16]
The most important virulence determinant in P. falciparum infection is the parasite´s ability to modify the surface of the infected red blood cell, thus creating an adhesive phenotype. The cytoadhesion is mediated through the P. falciparum erythrocyte membrane protein 1(PfEMP1) family, which is the product of var gene transcription. There is immense diversity in var genes in the parasite population, which has recently been a focus of research, due to its suggested association between increased transcription of specific var genes and the development of severe malaria.[6][17] The cytoadherence of mature-staged infected RBC to the endothelium, platelets, and uninfected red blood cells causes sequestration in the microvasculature of various organs, resulting in microcirculation obstruction, impaired tissues perfusion, lactic acidosis and consequently, end-organ damage.[18][19][20] Prominent sequestration occurs in the placenta during pregnancy, causing low birth weight, anemia, miscarriage, and congenital malaria.[6][19]
In essence, the key features that render a fatal disease are the sequestration of P. falciparum in tissues, in conjunction with the up-regulation of cytokines and other toxic substances and an absence or an untimely provision of effective antimalarial therapy.[6]
History and Physical
Malaria is a complex disease with a spectrum of clinical effects that not only differ between children and adults but can range from practically none in patients with asymptomatic parasitemia, to uncomplicated malaria, through to severe and possibly lethal malaria.[20] The mean incubation period for P. falciparum is 12 days, with most patients presenting in the first or second month after exposure in endemic areas.[21][22] It is key to take a detailed travel history in any patient with fever or history of fever, as malaria is a crucial diagnosis to consider in any individual who has traveled to a malaria-endemic area.[22][21]
Uncomplicated P. falciparum Malaria
Malaria can be separated into two disease presentations: uncomplicated and severe. [4] The WHO defines the presence of symptoms without clinical or laboratory signs to indicate severity or vital organ dysfunction as uncomplicated malaria.[6] Symptoms are generally non-specific, including fever, chills, myalgia, headache, anorexia, and cough, making clinical diagnosis unreliable.[4][21] Patients occasionally present with gastrointestinal symptoms, respiratory symptoms, and jaundice.[22] The classical malarial paroxysms with spiking fever, chills, and rigors occurring at specific intervals are relatively uncommon, but if present, indicate an infection with P. ovale or P. vivax.[3] Progression to severe or ultimately fatal disease is largely confined to P. falciparum infections, although only a small percentage, approximately 1% to 2% of infections, lead to severe malaria.[20][13] Features of a severe disease usually appear after 3 to 7 days of the abovementioned non-specific symptoms, although there are some reports of rapid deterioration, failure to recover consciousness after a grand-mal seizure, and non-immune patients dying within 24 hours of their first symptom.[13]
Severe P. falciparum Malaria
The patient presenting with at least one of the clinical or laboratory features listed below, with asexual P. falciparum parasitemia (either detected in the peripheral blood smear or confirmed with rapid diagnostic test) and no other confirmed cause of his symptoms, classifies as suffering from severe malaria.[13][23] Although P. falciparum is responsible for the majority of the cases of severe malaria, it is also, albeit rarely, observed with P. vivax and P. knowlesi infections.[18][13]
A shortened list of danger signs is used for rapid clinical assessment, which includes prostration, respiratory distress (acidotic breathing), and impaired consciousness.[4] Other clinical manifestations of severe malaria include multiple convulsions, radiologically confirmed pulmonary edema (respiratory failure due to acute lung injury progressing to acute respiratory distress syndrome), abnormal bleeding (disseminated intravascular coagulation), acute kidney injury, jaundice, shock, and coma.[4][21][13] Laboratory features in severe malaria can show severe anemia, hypoglycemia, acidosis, hyperlactatemia, renal impairment, and hyperparasitemia.[13] A comprehensive list of diagnostic criteria for falciparum malaria is shown in Table 1 - Diagnostic criteria for severe P. falciparum malaria.[13][18]
Physical Examination
Physical examination is usually unremarkable, especially of patients with uncomplicated malaria. They frequently present with irregular and erratic fever, reaching up to 41°C, sometimes accompanied by agitation or confusion.[3][13] Mild spontaneously resolving jaundice can sometimes be seen in patients with otherwise-uncomplicated falciparum malaria. [3][22] Other physical signs can include anemia and postural hypotension.[13] In some cases, patients can present with tender hepatosplenomegaly after some days. However, a palpable spleen is particularly common in otherwise healthy populations in endemic areas, reflecting repeated infections.[3] The comprehensive list of clinical features associated with severe malaria is shown in Table 1 - Diagnostic criteria for severe P. falciparum malaria.[13][18]
Children
Children are more likely to present with non-specific and gastrointestinal symptoms such as fever, lethargy, malaise, nausea, vomiting, abdominal cramps, and somnolence.[22] They are more likely to develop hepatomegaly, splenomegaly, and severe anemia without major organ dysfunction than adults. In a case of severe malaria, they present with more frequent seizures (in 60% to 80%), hypoglycemia, and concomitant sepsis but are less likely to develop pulmonary edema and renal failure than adults.[3][22]
Pregnant Women
The clinical features of infection in pregnancy vary from asymptomatic to severe, depending on the degree of (incomplete) immunity that a woman had acquired by the time she got pregnant. In semi-immune pregnant women, only a few infections result in fever or other symptoms.[15] Malaria in pregnancy has a devastating effect not only on maternal health but has been associated with increased infant mortality due to low birth weight caused by either intrauterine growth restriction or preterm labor or both.[15] P. falciparum infections are proven to be associated with complications such as maternal anemia, low birth weight, miscarriage, stillbirths, and congenital malaria.[6][15] It is more likely for a pregnant woman in the second or third trimester to develop severe malaria with complications such as hypoglycemia and pulmonary edema, compared to non-pregnant adults.[18]
Evaluation
Once malaria is considered a possible diagnosis, it is important to facilitate immediate laboratory testing.[4] It is essential to distinguish between non-falciparum and falciparum malaria.[24] As per the Centers for Disease Control and Prevention (CDC) guidelines, malaria should be routinely suspected in any febrile patient that has a recent history of travel to the endemic areas. The clinical features of either uncomplicated or severe malaria are non-specific, therefore requiring diagnosis by microscopy or rapid diagnostic test (RDT).[18] The results should be communicated back to the requesting doctor as soon as possible, ideally within a few hours.[24]
A full blood count, urea, creatinine and electrolytes, blood glucose level, and liver function tests should be routinely performed. Thrombocytopenia suggests both non-falciparum and falciparum malaria infections in non-immune adults and children. In severely ill patients, additional studies such as blood gases, blood culture, lactate, and clotting studies are appropriate. In patients with fever and impaired consciousness, one should consider a lumbar puncture to exclude meningitis.[24]
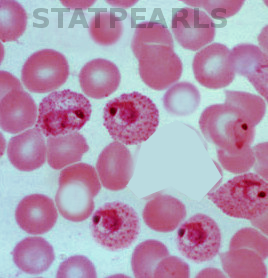
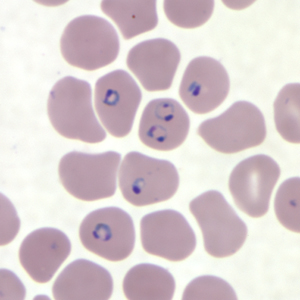
The golden standard for diagnosis is a microscopic analysis of thick and thin blood smears. Thick smears allow for a sensitive parasitemia quantification, as parasitemias as low as 30-50/microL can be detected, while thin smears enable a determination of the Plasmodium species, prognostic assessment based on the staging of parasite development and estimation of the proportion of neutrophils containing malaria pigment.[13][18] Three sets of thick and thin blood films spaced 12 to 24 hours apart should be performed by experienced laboratory personnel before a clinician can confidently rule out malaria.[8]
Perceived peripheral blood parasitemia varies greatly in patients with severe malaria, due to the sequestration of the infected red blood cells in tissues.[13][18] Although severe malaria can present with low parasite count, high counts are associated with increased risk of deterioration and subsequent treatment failure even without signs or symptoms of severity.[13] More than 2% of parasitized RBC suggests an increased chance of developing severe disease, and parasitemia over 10% is considered as one of the diagnostic criteria for severe disease and is associated with increased mortality.[24] Furthermore, in severe falciparum malaria, poor outcomes can be predicted by the presence of late-stage parasites in RBC and more than 5% of the neutrophils containing pigment.[18]
Rapid diagnostic tests are commonly used in addition to blood slides and are useful alternatives in settings where a microscopic diagnosis is non-reliable or infeasible.[24][9] It is, however, recommended that all the RDTs should be followed by microscopy for confirmation and, if positive, quantification of parasitemia.[9] They are immune-chromatographic tests that most commonly identify either malaria antigens (e.g., P. falciparum histidine-rich-protein 2 (PfHRP2)) or enzyme called Plasmodium lactate dehydrogenase (pLDH). Tests have several downfalls as they cannot provide quantitative results, can stay positive months after infection with P. falciparum, or are, if testing for pLDH, positive only while there are living parasites in the blood.[13]
Polymerase chain reaction (PCR) is one of the possible diagnostic modalities but is, even if promptly available, too timely to use for initial diagnosis and prompt treatment of acute malaria. It should, however, be used for research and epidemiologic purposes in any malaria infection in the USA, to determine and confirm the infecting species.[8] All cases should also be evaluated for evidence of drug resistance, as per CDC guidelines.[9]
Treatment / Management
CDC recommends that the treatment of malaria should not be initiated until the diagnosis has been confirmed by laboratory testing. Treatment should be initiated immediately after the confirmation of malaria infection. Empirical treatment is, however, reserved only for extreme cases where there is a strong clinical suspicion with convincing exposure history, presence of severe disease, or an inability to diagnose malaria due to inadequate laboratory facilities.
The treatment should be guided by three main factors: the infecting Plasmodium species, the clinical status of the patient, and drug susceptibility of infecting Plasmodium, determined by the geographic region where the infection was acquired. Chloroquine sensitivity can be expected if the infection was acquired in Central America west of Panama Canal, Haiti, and the Dominican Republic. In a case when the diagnosis is strongly suspected, and it cannot be confirmed, or when there is no species determination possible, given the global spread of P. falciparum resistant to chloroquine, the clinician should opt for a treatment option effective against chloroquine-resistant P. falciparum. CDC recommends making additional blood smears in infections with P. falciparum after initiating the treatment, to confirm adequate parasitological response (decrease in density) to treatment.
Extended, evidence-based, and comprehensive guidelines for malaria management and optimal dosing of antimalarial medications can be found in the third edition of WHOs Guidelines for the treatment of malaria. Treatment and management algorithms for malaria in the USA are available on the CDC´s official website.
Uncomplicated Falciparum Malaria
Uncomplicated falciparum malaria should be treated with one of the artemisinin-based combination therapies (ACT). Artemisinin-based combination therapy (ACT) is highly effective due to its effect on a broader range of parasite life cycles, causing faster parasite clearance and is therefore considered a drug of choice for uncomplicated malaria.[24] The duration of ACT treatment is 3 days. ACTs are also recommended for pregnant women in the second and third trimester but should be used during the first trimester only if other treatment options are not available. During the first trimester, a combination of quinine and clindamycin should be prescribed. In low transmission areas, gametocytocidal therapy (e.g., primaquine) is added to ACTs to reduce transmission potential (except pregnant women, infants younger than 6 months and women breastfeeding infants younger than 6 months).[18]
There are some other treatment options, although not as effective as ACTs, such as atovaquone-proguanil, quinine sulfate plus doxycycline, tetracycline, or clindamycin, and mefloquine. For chloroquine-sensitive P. falciparum infections (including
pregnant women), the drug of choice is chloroquine. However, any of the drug choices listed above for chloroquine-resistant strains can be used. Treatment options are the same for the pediatric population, with doses adjusted by the patient’s weight.
Severe Falciparum Malaria
All patients diagnosed or with a strong suspicion with signs and symptoms of severe malaria should be promptly treated with parenteral antimalarial therapy. Effective, urgent, and appropriate treatment has the greatest impact on prognosis.[24]
Intravenous or intramuscular artesunate is the first-line treatment in all patients (including children, lactating women, and pregnant women in all trimesters) worldwide and should be used for at least 24 hours and until the oral medication is tolerated.[18] Children should receive a higher dose (3 mg/kg BW per dose) of the artesunate to ensure the equivalent drug effect. The dose for larger children and adults is 2.4 mg/kg BW per dose. Three doses of intravenous artesunate should be given: one immediately, followed by a dose at 12 hours and 24 hours.
If artesunate is not available, the recommended drug is artemether in preference to quinine. After the complete course of a 24 hour intravenous artesunate, a regimen of a follow-up drug should be completed. A full 3-day regimen of ACT is recommended. In returning traveler, the follow-on antimalarial medication needs to be other than the antimalarial medication taken for prophylaxis.[18]
Intravenous artesunate is currently not approved by the Food and Drug Administration (FDA) or commercially available in the U.S. but is available from CDC under an expanded-access investigational drug (IND) protocol. It is prepositioned at distribution sites throughout the USA and readily available after contacting CDC. Since severe malaria can progress rapidly, CDC offers guidance on oral treatment that can be used while waiting for IV artesunate to be delivered. They recommend interim treatment with oral artemether-lumefantrine (preferably), atovaquone-proguanil, quinine sulfate, or mefloquine. If a patient can not tolerate oral medications, administration after an antiemetic or via nasogastric tube should be considered.
Patients with falciparum malaria should be admitted to the hospital due to the possibility of deterioration even after the effective treatment was initiated. If patients are determined to have uncomplicated malaria, they can be treated as outpatients after confirming that they can tolerate oral therapy, and the parasitemia has declined.[13] Ideally, a patient with severe malaria should be admitted to an intensive-care unit or high dependency unit with close monitoring of clinical status, vital signs, consciousness level, and laboratory values.[13][24]
It is important to provide supportive therapy, such as glucose to maintain euglycemia and acetaminophen for fever control, careful, individualized fluid management as patients present with variable degrees of hypovolemia, acidosis, and acute kidney failure. Blood transfusion is sometimes indicated in severe anemias; benzodiazepines should be used for seizure control. However, prophylactic antiepileptic medications are not recommended, and empiric antibiotics should be used in children with severe malaria and adults with concurrent shock.[4][22][18]
Differential Diagnosis
Other travel-related infections such as typhoid, viral hemorrhagic fevers (such as Ebola, Lassa fever, etc.), hepatitis, dengue, and other arboviruses, enteric fever, avian influenza, tuberculosis, MERS-CoV infections, HIV, meningitis, and encephalitis can resemble malaria. Non-tropical infections such as bacterial pneumonia, septicemia, and influenza should be excluded.[24][25]
Cerebral malaria can mimic bacterial meningitis, measles, (locally prevalent) viral encephalitis, toxic syndromes, and intracranial vascular or mechanical events.[13]
Prognosis
Patients with uncomplicated malaria, especially with timely diagnosis, treatment, and proper compliance, usually recover from malaria without consequences. The mortality rate for uncomplicated malaria is low, around 0.1%.[3][6] The mortality rate rises steeply once the patient develops signs and symptoms of severe falciparum malaria. Adults have higher mortality rates and more frequent multisystem involvement than children, with mortality rates being 18.5% and 9.7%, respectively.[20] The two main determinants reflecting the outcome for both adults and children were found to be the level of consciousness assessed by coma scales and the degree of metabolic acidosis, assessed clinically by breathing pattern or more precisely with measurement of bicarbonate, base deficit, and plasma lactate.[13] While the general mortality of treated severe malaria is between 10% to 20%, the mortality in pregnant women reaches approximately 50%.[18]
Complications
A distinct complication of P. falciparum malaria is cerebral malaria (CM), a diffuse and symmetrical encephalopathy.[16] It is a clinical syndrome defined as an impaired consciousness (clinically determined as stated in Table 1) that cannot be attributed to other causes such as convulsions, hypoglycemia, sedative drugs, or other non-malarial causes and is associated with an unequivocal diagnosis of malarial infection. Due to several other possible causes of altered consciousness, the presence of retinopathy has been used in an attempt to increase the specificity of diagnosis of CM and improve the classification of severe malaria.[13]
Other possible complications, predominantly caused by P. falciparum, include:
- Acute kidney injury complicates up to 40% of P. falciparum malaria.[18]
- Non-cardiogenic pulmonary edema, acute respiratory distress syndrome (ARDS), and hypoxia[13]
- Electrolyte and fluid abnormalities[13]
- Acid-base disturbances – mostly acidosis and hyperlactatemia[13]
- Hypoglycemia, often exacerbated by quinine therapy[4]
- Anemia, with the reduction in hemoglobin levels being proportional to disease severity and duration of illness before treatment[13][16]
- Other hematological complications (delayed hemolytic anemia following artemisinin treatment, hyper-reactive malarial splenomegaly (HMS), and splenic rupture)[4]
- Blackwater fever and hemoglobinuria due to intravascular hemolysis in patients with severe clinical manifestations of falciparum malaria[13]
- Profound thrombocytopenia is often associated with P. falciparum infection, however, bleeding and disseminated intravascular coagulation (DIC) are rare.[13]
- Hepatic dysfunction[13]
- Shock and associated infections (such as Salmonella bacteriemia, aspiration pneumonia, nosocomial infections, etc.)[3][13]
- Neurological sequelae (epilepsy, permanent focal deficits, etc.)[4]
The three most common complications occurring in children are cerebral malaria, severe anemia, and acidosis, either isolated or overlapping.[20]
Deterrence and Patient Education
Although no intervention for preventing the infection is 100% effective, there are several different approaches available that can be used alone or in combination. Personal protective measures reduce the risk of exposure to infective mosquitoes, and chemoprophylaxis can aid in reducing the risk of a poor outcome if infected.[26] A common approach usually applied is an “ABCD” of malaria – A standing for awareness of the risk, B for bite avoidance, C for compliance with chemoprophylaxis, and D for diagnosis in case of fever. It is essential to consider traveler´s health (especially pregnancy, age, immunosuppression) when assessing the risk for developing severe malaria and choosing an appropriate antimalarial drug.[10]
It is necessary to emphasize the importance of personal protective measures such as barrier clothing, insecticide-impregnated bed nets, application of effective mosquito repellent (higher percentage of active ingredient provides longer protection), and spraying the residence with insecticide. Products that contain 20%-40% of DEET (N, N-Diethyl-meta-toluamide), picaridin, oil of lemon eucalyptus or PMD (p-menthane-3,8-diol) and IR3535 are recommended. Behaviors to minimize exposure to mosquitos are also encouraged – for instance, staying indoors from dusk till dawn, choosing screened accommodations.[9][26] Indoor residual spraying and long-lasting insecticidal nets remain to be the most effective tools for malaria control and elimination, despite the emergence of insecticide-resistant Anopheles mosquitos.[27][28]
A standard recommendation for all travelers to endemic areas is strict compliance with the antimalarial drug. There are several different drug choices, prescribed after assessing individual´s risk (level of local transmission, duration of exposure, rural vs. urban travel, type of travel and season), travelers health status, present contraindications, level of parasite drug resistance (mostly chloroquine and mefloquine resistance) and traveler’s preference based on schedule, cost and nature of possible side effects. There is an increasing problem with counterfeit and substandard medications being sold in several Sub-Saharan countries, and travelers should be advised to buy the needed medications before departure.[29][10] A complete list of recommendations for drug choice can be found on the CDCs website – Malaria Information by Country.
As pregnant women are at increased risk for severe malaria, the WHO and other organizations recommend not traveling to the endemic areas.[10]
Travelers to the endemic areas, former residents of malaria-endemic areas and people diagnosed with malaria should be informed, as per Food and Drug Administration (FDA) recommendations, that they may not donate blood for 1 year after travel or 3 years after departing or revisiting the country or for 3 years after treatment, respectively.[9]
Travelers should be informed that malaria can be fatal with delayed treatment, and one should, therefore, seek medical attention while abroad if symptoms of malaria develop and not fly back for treatment, as medical treatment may not be readily available on transit.[9]
Pearls and Other Issues
Malaria vaccine would be an ideal tool to control, prevent, eliminate, and ultimately eradicate the disease. The complexity of P. falciparum infection has hindered several attempts at developing an effective vaccine, yet the acquisition of partial immunity and successful treatment of malaria with purified immunoglobulins from semi-immune adults showed that the development of a vaccine is attainable. Researchers have identified several potential targets in the parasite´s infectious cycle. Currently, 20 vaccine candidates are undergoing clinical trials.[27] The most promising and most advanced vaccine to be developed so far is a licensed RTS, S subunit vaccine (RTS, S/ASO1), targeting the pre-erythrocytic stage of infection, thus preventing hepatocyte infection and parasite development, consequently limiting RBC invasion. It consists of a recombinant protein of the P. falciparum circumsporozoite protein (CSP) conjugated to the hepatitis B surface antigen.[27] RTS, S/ASO1, the first malaria vaccine which has received regulatory approval for human use, was used to start the first routine malaria vaccination program in Africa – a pilot study in Malawi in April 2019, which has since expanded to Kenya and Ghana. The pilot study is planned to last about 50 months and to enroll 720 000 children in vaccination and control clusters, however several safety concerns, that are now being investigated in the pilot study, have already been identified in phase III trials: higher risk of meningitis, cerebral malaria and doubled female mortality. If no serious safety concern is determined in the first 24 months of trial, the pilot study will run its full course before any decision will be made about its broader use in endemic countries.[30]
There has been an emergence of a (widely used) pyrethroid insecticide-resistant malaria vector population, causing extensive research with their first results suggesting a better effect of long-lasting insecticidal nets treated with PBO (piperonyl butoxide) instead of pyrethroid laced nets.[28]
Enhancing Healthcare Team Outcomes
Most cases of malaria in the U.S. are imported and could, therefore, be avoided with appropriate personal protective measures and compliance with prescribed chemoprophylaxis. Health care providers on all levels must be educated not only on the diagnosis and treatment of malaria but especially on the importance of prevention. They should be able to provide enough information to travelers at risk. Travelers to the endemic areas should obtain appropriate clothing, insecticides, and other protective gear based on the information offered by health care providers and CDC. Collaboratively, a physician and a pharmacist, should decide on, recommend and prescribe appropriate chemoprophylaxis, considering traveler's personal preferences, time of travel, current health status, risk factors, drug-drug interactions, and possible contraindications, while emphasizing the importance of compliance with the drug-taking regimen.
The crucial issue in the diagnosis and treatment of malaria is the consideration of the possibility of diagnosis. Expert advice from an infectious disease or travel medicine specialist should be sought once malaria is a suspected diagnosis, especially in the setting of severe disease.[24] The execution of a microscopic analysis of a stained blood smear often depends on several factors such as laboratory equipment, quality of reagents, and laboratorian expertise. Laboratorians should, therefore, be trained in the preparation and analysis of blood-smears to diagnose a malarial infection correctly. The results need to be communicated back to the physician as soon as possible. All laboratory-confirmed cases of malaria should be reported to CDC to help with their surveillance efforts. Healthcare providers that are a part of the interprofessional team (nurses, respiratory therapists, physicians, etc.) managing patients with severe malaria in critical or intensive care units, should be educated on early recognition of complications.
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)