Pulmonary Function Tests
- Article Author:
- Mario Ponce
- Article Editor:
- Sandeep Sharma
- Updated:
- 9/2/2020 7:30:51 PM
- For CME on this topic:
- Pulmonary Function Tests CME
- PubMed Link:
- Pulmonary Function Tests
Introduction
Pulmonary function tests (PFTs) allows physicians to evaluate the respiratory function of their patients. They are reproducible and accurate. Ultimately, the results of the PFTs are affected by the effort of the patient. PFTs do not provide a specific diagnosis, but together with the history, physical exam, and laboratory data help clinicians reach a diagnosis. PFTs also allow physicians to quantify the severity of the pulmonary disease, follow it up over time, and assess its response to treatment.[1]
Procedures
Spirometry
Spirometry is a physiological test which measures the ability to inhale and exhale air in relation to time. Spirometry is a screening test of general respiratory health. The main results of spirometry are forced vital capacity (FVC) and forced expiratory volume (FEV). The procedure of spirometry has 3 phases: 1) maximal inspiration; 2) a “blast” of exhalation; 3) continued complete exhalation to the end of the test. There are within-maneuver acceptability and between-maneuver reproducibility criteria for spirometry (Table 2).
Vital capacity (VC) is the volume of gas expelled from full inspiration to residual volume. The FVC is similar, but the patient is exhaling at maximal speed and effort.
The FEV is the forced expiratory volume in t seconds from a position of full inspiration. Forced expiratory volume in the first second (FEV)) is used to classify the severity of obstructive lung diseases. The reversibility testing is administered using a bronchodilator (short-acting beta 2-agonist or anticholinergic agent). An increase in either FEV or FVC of 3^12% and 3^200 mL is considered positive bronchodilator response. A lack of a response does not predict lack of response to bronchodilators. The patient should hold their bronchodilators before the reversibility testing.
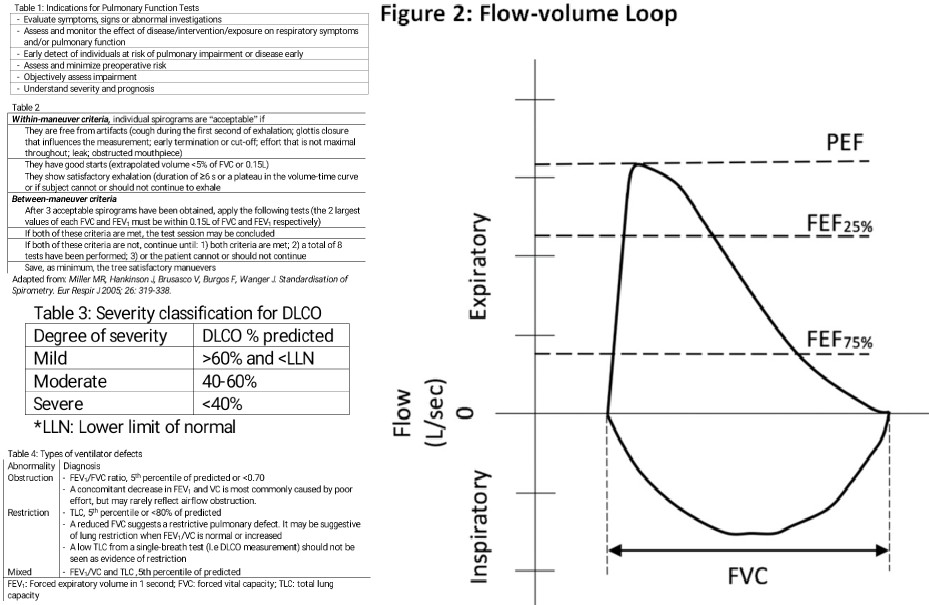
The maximal flow-volume curves are a great asset to detect mild airflow obstruction (Figure 2). There is an inspiratory and expiratory loop. [2]
Lung Volume
The key measurement of lung volumes is functional reserve capacity (FRC). Once FRC has been measured, all other volumes can be calculated. FRC is the volume of the amount of gas in the lungs at the end of expiration during tidal breathing. FRC is the sum of expiratory reserve volume (ERV) and residual volume (RV). ERV is the volume of gas maximally exhaled after end-inspiratory tidal breathing. RV is the volume of gas in the airways after a maximal exhalation.
In addition to RV and ERV, there is tidal volume (TV) and inspiratory reserve volume (IRV). IRV is the volume of gas that can be maximally inhaled from the end-inspiratory tidal breathing. TV is the volume of gas inhaled or exhaled with each breath at rest.
There are 2 methods to measure lung volumes: body plethysmography and gas dilution methods (nitrogen washout and inert gas dilution). Gas dilution method uses an inert gas (poorly soluble in alveolar blood and lung tissues), either nitrogen or helium. The subject breathes a gas mixture until equilibrium is achieved. The volume and mixture of gas exhaled after the equilibrium have been achieved permit the calculation of FRC. In body plethysmography, the subject sits inside a body box and breathes against a shutter valve. FRC is calculated using Boyle Law (at a given temperature, the product of gas volume and pressure is constant). FRC calculated by body plethysmography is usually larger in subjects with obstructive lung disease and air trapping than FRC calculated using gas dilution methods. Body plethysmography is considered the gold standard for lung volumes measurement.
Capacities are the sum of 2 or more volumes (Figure 1). TLC is the gold standard for diagnosis of restrictive lung disease. TLC less than 5 percentile of predicted or less than 80% predicted are diagnostic of a restrictive ventilatory defect.[3][4]
Diffusion Capacity
Diffusion studies the diffusion of gases across the alveolar-capillary membranes. Its measurement uses carbon monoxide (CO) to calculate the pulmonary diffusion capacity. The most common method is the standard single-breath D. It is measured in milliliters per minute per mm Hg.
The factors affecting the D are volume and distribution of ventilation, mixing and diffusion, the composition of the gas, characteristics of the alveolar membrane and lung parenchyma, the volume of alveolar capillary plasma, concentration and binding properties of hemoglobin and gas tensions in blood entering the alveolar capillaries. A detailed list of factors affecting DLCO is listed in Table 3.
The diffusion capacity depends on multiple factors, and its value should be adjusted. Specific adjustments should be made for hemoglobin, carboxyhemoglobin, and FiO for a correct interpretation. Adjustment for lung volumes is controversial, and further studies are needed.
The DLCO is interpreted in conjunction with spirometry and lung volumes. Table 3 shows the severity classification for DLCO. For example, high DLCO is associated with asthma, obesity and intrapulmonary hemorrhage. Normal spirometry and lung volumes with low DLCO can be present in pulmonary vascular diseases, early ILD or emphysema. An obstructive ventilatory defect with low DLCO suggests emphysema or lymphangiomyomatosis.[5][6]
Respiratory Muscle Pressures
The respiratory muscle strength is assessed with the maximal inspiratory pressure (MIP); also called negative inspiratory force (NIF) and maximal expiratory pressure (MEP). The MIP reveals the strength of the diaphragm and other inspiratory muscles; whereas, the MEP indicates the strength of the abdominal and other expiratory muscles.
MIP and MEP are measured three times, maximal value is reported. For adults 18 to 65 years old, MIP should be lower than -90 cmHO in men and -70 cmHO in women. In adults older than 65 years old, MIP should be less than -65 cmH2O in men and -45 cmH2O in women. MEP should be higher than 140 cmH2O in men and 90 cmH2O in women. MEP less than 60 cmH2O predicts a weak cough and difficulty clearing secretions.
Central and Upper Airway Obstruction
Central or upper airway obstruction (UAO) could occur in the extrathoracic (pharynx, larynx, and the extrathoracic part of the trachea) and intrathoracic airways (intrathoracic trachea and main bronchi). The FEV and/or FVC are not affected by this type of obstructions, but the peak expiratory flow (PEF) can be severely decreased. An FEV/PEF ratio of greater than 8 suggests central or UAO.
Three maximal and repeatable forced inspiratory and expiratory flow-volume curves are necessary. It is key that efforts, both inspiratory and expiratory are maximal.
The maximal inspiratory flow is largely decreased with an extrathoracic airway obstruction because there is a negative pressure inside the airways during inspiration. Inspiratory flows are not affected by intrathoracic lesions; the negative pleural pressure maintains the intrathoracic airways open. The maximal expiratory flow (peak flow) is decreased with intrathoracic and extrathoracic lesions.
The obstructions can be intrathoracic or extrathoracic and variable or fixed. In summary, fixed obstructions will have decreased inspiratory and expiratory flows; variable obstruction will depend on the location (intrathoracic or extrathoracic). The flow-curve is not a sensitive test; the absence of the classic pattern does not rule out a central or UAO.[1][7][8][9]
Indications
There are multiple indications to obtain PFTs. Table 1 summarizes the most commons indications.
PFTs can be physically demanding for patients, and it is recommended to wait one month after an acute coronary syndrome or myocardial infarction. Other relative contraindications are thoracic/abdominal surgery, brain/eye/ear/otolaryngological surgery, pneumothorax, ascending aortic aneurysm, hemoptysis, pulmonary embolism, severe hypertension (SBP greater than 200 mm Hg, DBP greater than 120 mm Hg).[7]
Normal and Critical Findings
Normal findings of spirometry are FEV/FVC ratio of greater than 0.70 and both FEV and FVC above 80% of predicted value. If lung volumes are performed, TLC above 80% of predictive value is normal. Diffusion capacity above 75% of predicted value is considered normal as well.
Critical findings would be severe ventilator defects.
Interfering Factors
The results of the PFTs might not be accurate if there is lack of cooperation or poor understanding of instructions from the patient. Also, if the patient has an acute illness or symptom (for example, altered mental status, nausea, diarrhea, abdominal or chest pain) are likely to have suboptimal results.
Complications
PFTs are safe in general, and there are no complications. There is some potential harm from 4 key factors:
- Maximal pressures generated in the thorax and their impact on abdominal and thoracic organs/tissues
- Large swings in blood pressure causing stresses on tissues in the body
- Expansion of the chest wall and lungs
- Spread of infections (e.g., tuberculosis, hepatitis B, HIV)
Contraindications of PFTs are related to those 4 factors to prevent potential complications like acute coronary syndrome, rupture of aneurysms, and dehiscence of the surgical wound.[7]
Clinical Significance
The first step to interpret PFTs is to review the test quality. Once the quality has been assured, then comparisons can be made with healthy subjects and abnormal physiological patterns.
Types of Ventilatory Defects (Table 3)
Obstructive Abnormalities
An obstructive defect is a disproportional decrease in maximal airflow from the lung (FEV) in relation to the maximal volume (FVC) that can be displaced from the lung. In practical terms, an FEV/FVC ratio of less than 0.70 defines an obstructive ventilatory defect
The earliest changed associated with an obstruction in small airways is a slowing in the terminal portion of the spirogram (mid-flows). Qualitatively, it is reflected by a concave shape on the flow-volume curve. Quantitatively, proportionally increased the reduction in the instantaneous flow measured after 75% of the FVC has been exhaled (FEF) or FEF. Nevertheless, mid-flow abnormalities are not specific to airflow obstruction, and it is no longer a diagnostic criterion of airflow obstruction.
Measurement of lung volumes is not mandatory to identify obstructive ventilatory defects. Lung volumes are helpful when the FEV and FVC are equally decreased, and FEV/FVC ratio is normal or almost normal. The pattern usually reflects the failure of the patient to completely inhale or exhale. The flow-volume curve should be concave. Additionally, the TLC should be normal and RV abnormally increased.
The severity of obstructive defects is based on the FEV percentage predicted value. The American Thoracic Society classification is as follows:
- Mild (FEV percentage predicted greater than 70%)
- Moderate (FEV percentage predicted 60% to 69%)
- Moderately severe (FEV percentage predicted 50% to 59%)
- Severe (FEV percentage predicted 30% to 49%)
- Very severe (FEV percentage predicted less than 35%)
Restrictive Abnormalities
Restrictive ventilatory defects are characterized by a normal FEV/FVC ratio (3^70%) and reduction in TLC below the fifth percentile or 80% of the predicted value. They can be suspected if the FVC is reduced (less than 80%), FEV/FVC is increased (greater than 85% to 90%), and the flow-volume curve shows a convex shape. Spirometry can only suggest a restrictive defect; lung volumes are necessary to confirm the defect. As mentioned previously, TLC is the gold standard for the diagnosis of a restrictive defect. The severity of the restrictive defects is based on the FEV percentage predicted value, using the same severity scale as obstructive defects.
Mixed Abnormalities
Characterized by the presence of an obstructive and restrictive defect. Diagnosed when both FEV/FVC and TLC are below the five percentile of their predicted values (or FEV/FVC ratio less than 0.70 and TLC less than 80% predicted value). If FEV1/FVC ratio is low and FVC is less than 80% of predicted, but TLC is normal, the patient has an obstructive pattern, and FVC is low due to hyperinflation. If the TLC is low, the patient has a mixed obstructive and restrictive pattern. For mixed defects, spirometry and lung volumes are needed for diagnosis. The severity of the restrictive defects is also based on the FEV percentage predicted value, using the same severity scale as obstructive defects.[7][3]