Anatomy, Head and Neck, Salivary Glands
- Article Author:
- Mousa Ghannam
- Article Editor:
- Paramvir Singh
- Updated:
- 8/16/2020 2:11:10 AM
- For CME on this topic:
- Anatomy, Head and Neck, Salivary Glands CME
- PubMed Link:
- Anatomy, Head and Neck, Salivary Glands
Introduction
The salivary glands are an important set of exocrine glands that functions to produce, modify and secrete saliva into the oral cavity. They glands are divided into two main types: the major paired salivary glands, which includes the parotid, submandibular and sublingual glands, and the minor salivary glands, which line the mucosa of the upper aerodigestive tract and the overwhelming entirety of the mouth.[1]
Human salivary glands produce anywhere from 0.5 to 1.5 L of saliva daily, which has many different functions in the oral cavity. This includes lubrication of food to support mastication and swallowing, lubrication of the buccal mucosa to facilitate proper speech and provision of an aqueous medium, which is necessary to experience taste. It also facilitates digestion of triglycerides and starches via the secretion of lipases and amylases, respectively, and plays a protective role against infections via its many protective organic constituents. These include the secretory piece, a glycoprotein that forms a complex with immunoglobulin A (IgA) to defend against viruses and bacteria, lysozymes that cause bacterial agglutination, autolysin to degrade bacterial cell walls and lactoferrin to sequester iron, an element vital to bacterial growth.[2] Additionally, saliva contains ionic compounds, such as bicarbonate, which buffer acids produced by bacteria and protects the oral cavity and esophagus from gastric juice. As a result, saliva plays an extremely important role in protecting the mouth from chronic buccal mucosal infections and dental caries.[3]
Structure and Function
Anatomical Location
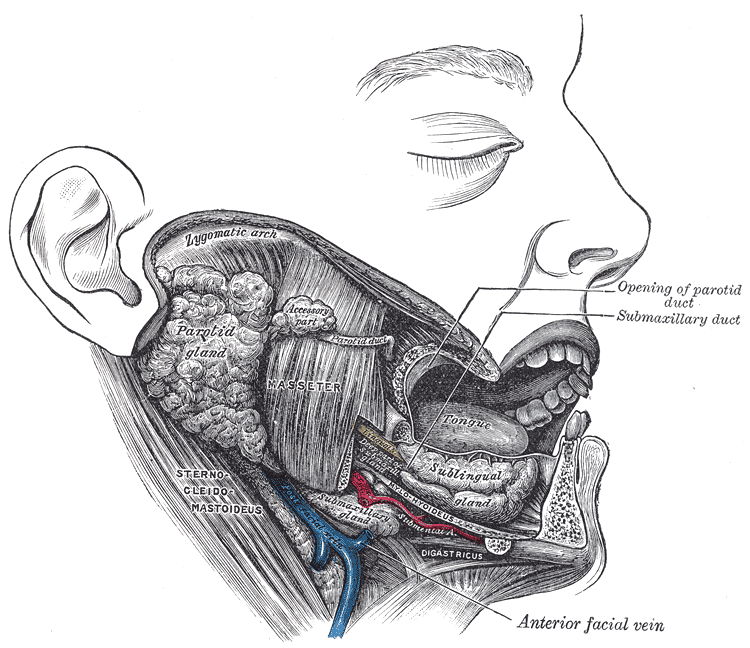
The parotid gland (PG) is the largest of the three major salivary glands and is located between the sternocleidomastoid muscle and the masseter, extending from the mastoid tip to just below the angle of the mandible. A small tail projects off the inferior edge of the gland, separated by the submandibular space only by the stylomandibular ligament. Going from superficial to deep, the gland is superficial to the facial nerve, an area of important surgical relevance, followed by the retromandibular vein and the external carotid artery. Stenson’s duct, the main excretory duct of the PG, projects from the anterior portion of the gland over the masseter, piercing the buccinator muscle to enter the oral cavity in the buccal mucosa near the second maxillary molar.[4]
The submandibular gland (SMG), the second largest gland, is about one half the weight of the parotid and is found inferior to the mandible, between the anterior and posterior bellies of the digastric muscle. The SMG has a smaller anterior lobe and a larger posterior lobe that connect with each other around the posterior free edge of the mylohyoid muscle. The main excretory duct, referred to as Wharton’s duct arises from the smaller, deep lobe inferior to the mucosa of the floor of the mouth to enter the oral cavity along the lateral side of the frenulum linguae at the sublingual caruncle. The hypoglossal nerve runs parallel and inferior to Wharton's duct.[4]
Directly lateral to the duct lies the sublingual gland (SLG), the last of the major salivary glands. It lies submucosally in the floor of the mouth, immediately posterior to the anterior lobe of the submandibular gland. Relative to the muscles, it is located directly superior to the mylohyoid, between the mandible and genioglossus muscles. Medially, between the base of the tongue and the sublingual gland are the submandibular duct and the sublingual nerve, while, laterally, it is adjacent to the sublingual groove on the medial side of the mandible. Rather than having one main duct, it contains a series of short ducts that project directly into the floor of the mouth and also into Wharton’s duct.[4][5]
While the major salivary glands produce 92% to 95% of saliva, the remainder is made by roughly 600 to 1000 minor salivary glands. They can be found in almost any part of the mouth (except for the gingiva and anterior part of the hard palate), but present most commonly in the lips, buccal mucosa, palate, and the tongue. Each gland has exactly one duct, and although it contains both serous and mucous acini, their secretion is mainly mucous. Unlike the major salivary glands, they are not encapsulated by connective tissue but merely surrounded by it. Von Ebner glands, a subset of the minor salivary glands, are found surrounding the circumvallate papillae on the dorsal surface of the tongue. They secrete a serous fluid that helps with lipid hydrolysis and also play a role in taste perception.[1]
Architecture and Function
Salivary glands contain three major cell types: acinar cells, ductal cells, and myoepithelial cells. Despite their different locations, each of these glands shares the same fundamental branched ductal structure that opens into the oral cavity with glandular secretory end pieces, acini, that make saliva. Myoepithelial cells display 4 to 8 processes that wrap around the acini and intercalated ducts, rhythmically contracting to squeeze saliva from the acinar units upon stimulation by nerves, through the duct system, and into the oral cavity. In addition to myoepithelial cells, acinar cells are also surrounded by an extracellular matrix, immune cells, stromal cells, myofibroblasts, and nerve fibers.[6]
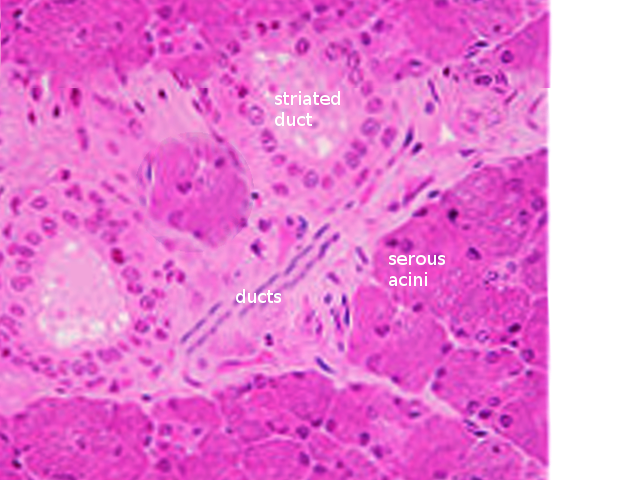
There are three main types of acini: serous, mucinous and seromucous. Serous acini have a relatively spherical morphology and produce a watery secretion containing proteins that are modified and stored in secretory, or zymogen, granules abundant at the apex of the cell. In contrast to serous acini, mucinous acini store a significantly more viscous secretion that is rich in glycoproteins (or mucins), which becomes hydrated upon exocytosis to form mucus. Lastly, seromucous acini contain secretions of both types, although one type of secretion may predominate. Each of the major salivary glands contains different proportions of each acinus, affecting the composition of secretions that each produces. The parotid contains mostly serous acini and makes mostly serous fluid. Although submandibular gland contains both serous and mucinous acini, it is predominantly serous with only 10% of acini being mucinous. These mucinous acini contain crescent-shaped, serous demilune cells that wrap around the top of the cells. Similarly, the sublingual glandular tissue has mixed acini and demilunes but is predominately mucinous instead.[7]
An integral function of the ductal system is modifying the electrolyte content of the secretory product as it passes through the ductal system. Ductal structures are categorized and differentiated by their cellular composition, location and functional properties forming three main types: the intercalated, striated and excretory ducts. The intercalated ducts project directly from the acini and are formed by a single layer of cuboidal cells. Here, bicarbonate is secreted while chloride is absorbed from saliva. The intercalated ducts empty directly into the striated ducts, which are comprised of columnar cells with deep basolateral membrane invaginations that give it its characteristically striated appearance. These cells function mainly in reabsorption of sodium and the addition of potassium to the secretory product. The excretory duct has the largest diameter, and its epithelial lining is comprised of pseudostratified columnar cells. At this point, salivary fluid is relatively hypotonic to plasma because the rates of sodium and chlorine resorption are greater than that of potassium and bicarbonate secretion within the ducts. The luminal accumulation of ions within the ductal system creates an osmotic gradient responsible for the passive travel of water throughout the ducts.[8][9]
Embryology
Salivary glands development begins at around 6 to 8 weeks when reciprocal interactions between the epithelium and the adjacent mesenchyme initiate thickening of the oral ectoderm. The glands develop via the process of branching morphogenesis of epithelium. This branching process, which occurs in other organ systems including the lungs, kidneys, and mammary glands, features systematic proliferation, clefting, differentiation, migration, and cell death while engaging in bi-directional signaling between the developing mesenchyme, endothelial cells, and nerves.[10] The terminal buds at the end of the branched ductal structures become acini at 14 weeks. While the parotid is the first to begin its formation, development of lymphatic tissue throughout the gland makes it the last of the glands to be enclosed in connective tissue and the only salivary gland with an enclosed lymphatic system. By 13 to 16 weeks, human SMGs are well-differentiated, featuring microvilli and desmosomes from cells near the lumens. The basal lamina surrounds the epithelium, and myoepithelial cells are thought to begin to appear at this stage. After 16 weeks, striated and intercalated ducts can be noticed. The glands stop developing at 28 weeks, marking the point at which acini produce secretory products. The glands are fully functional at birth.[11][12]
Blood Supply and Lymphatics
Parotid
Blood is supplied to the parotid gland from the external carotid artery, which flows superiorly from the carotid bifurcation and alongside the posterior aspect of the digastric muscle. This artery courses medially to the parotid, bifurcating into the superficial temporal artery, which flows superior to the parotid to the scalp, and the maxillary artery, which travels from the medial side of the parotid to carry blood to the infratemporal fossa and the pterygopalatine fossa. Lastly, the transverse facial artery branches off of the superficial temporal artery, projecting anteriorly between the zygoma and parotid duct to provide blood to both the parotid gland and parotid duct, as well as the masseter muscle. Blood from the maxillary vein and the superficial temporal vein join together to form the retromandibular vein, which runs through the parotid anterior to the facial nerve to join the external jugular vein. The retromandibular vein displays significant variation in its anatomy, and in some cases splits into an anterior and posterior branch. The anterior branch can form a union with the posterior facial vein, forming the common facial vein. The posterior facial vein lies directly inferior to the marginal mandibular branch of the facial nerve and is often used as a landmark for pinpointing the nerve branch. Contrastingly, the posterior branch of the retromandibular vein can join with the post-auricular vein to drain into the external jugular vein.[13]
The lymphatic system of the parotid gland differs from the other major salivary glands, in that there is a high density of lymph nodes in and around it. In this way, it contains two nodal layers, draining into both the superficial and deep cervical lymph systems. Roughly 90% of the nodes are found in the superficial layer between the glandular tissue and its capsule.[14]
Submandibular
Blood to the submandibular gland is supplied mainly from the facial artery, a branch of the external carotid artery. This artery proceeds medial to the posterior belly of the digastric muscle, to project deep through the gland capsule before crossing the inferior margin of the mandible, referred to as the facial notch. After this point, it proceeds superiorly adjacent to the inferior branches of the facial nerve and into the face.[15]
Unlike the parotid, the pre-vascular and post-vascular lymph nodes of the submandibular gland are found between the gland and its fascia, as opposed to being located within the glandular tissue. These nodes are closely associated with the facial artery and vein at the superior portion of the gland, and exit into the deep cervical and jugular chains.[15]
Sublingual
The sublingual gland receives blood from the submental and sublingual arteries, which are branches of the lingual and facial arteries, respectively. While deoxygenated blood is carried away by veins parallel to the arteries mentioned above, drainage occurs predominantly through the submandibular lymph nodes.[16]
Nerves
Both the parasympathetic and sympathetic nerves innervate salivary glands, with parasympathetic stimulation leading to serous salivary product and ion secretion, and sympathetic stimulation leading to increases in the secretion of proteins. The latter is also known to be involved in regulating the blood flow of the glands, as well as local inflammatory and immune mediators.[17][18]
The parotid gland is innervated via the glossopharyngeal nerve (CNV-IX), relaying preganglionic parasympathetic fibers from the inferior salivatory nucleus in the medulla to the otic ganglion. Postganglionic fibers from the OG then join the auriculotemporal nerve of the cranial nerve (CNV-VIII) to innervate the parotid. The submandibular ganglion (SG), located within the SMG and adjacent to the lingual nerve, contains the cell bodies of parasympathetic fibers that innervate the SMG and SLG, as well as their myoepithelial cells.[19] The preganglionic parasympathetic fibers of the SMG and SLG originate in the superior salivatory nucleus in the pons, eventually joining with the facial nerve (CNV-VII). These fibers pass through the chorda tympani, out the skull through the petrotympanic fissure, and join with the lingual nerve, which runs directly adjacent to Wharton’s duct, to finally synapse in the SG.[18]
Contrastingly, the cell bodies of the sympathetic fibers are found in the superior cervical ganglion in the neck, where postganglionic fibers innervate the glands along blood vessels branching off of the carotid plexus. The preganglionic fibers originate in the thoracic ganglion, where they ascend through the spinal cord.[19]
Secretion by the minor salivary glands are not regulated by neuronal control like the major glands are. Instead, they continuously secrete small amounts of saliva, even at night when the major glands are resting, resulting in sustained comfort from lubrication of oral surfaces.[20]
Physiologic Variants
With respect to the parotid gland, roughly 20% of individuals have accessory parotid tissue along the anterior masseter muscle. This is often confused with a mass in PET imaging, but on rare occasions can be due to any pathology affecting the parotid, such as neoplasms. Other anatomic variants of the parotid include unilateral and bilateral aplasia, parotid duct duplication, and parotid duct fistulas characteristic of branchial cleft anomalies. Accessory tissue is histologically distinguished from normal parotid by containing mucinous acinar cells in addition to the serous acinar cells.[21]
Surgical Considerations
Surgical excision and resection of the salivary glands are a common choice of treatment for many pathological conditions. This is especially the case for parotidectomy and submandibulectomy in addressing benign and malignant tumors. A thorough understanding of craniofacial anatomy is essential in treating these conditions, and surgical approach should focus on prudent identification of the facial nerve.[22]
In the case of parotidectomies, the goal of the surgery is centered on carefully removing the tumor and the associated tissue margins of the parotid, while protecting the function of the facial nerve if the nerve is not directly associated. Anatomical landmarks for identifying the facial nerve trunk are therefore critical, with some being more effective than others. For example, landmarks demonstrating significant variabilities, such as the posterior digastric muscle or those that are difficult to localize and or symmetrical, such as the tragal pointer, are less consistently reliable. Contrastingly, bony landmarks are known to be among the most reliable, and the tympanomastoid fissure has been known to be one of the safest and most reliable landmarks for identifying the facial nerve trunk. Other methods for identification of the nerve trunk include retrograde tracing of its distal branches proximally.[22]
Compared to surgical removal of the parotid, removal of the submandibular gland and sublingual gland is relatively more straightforward and necessitates a deep understanding of the anatomy of the mouth floor and the neck.[23]
Clinical Significance
General Considerations
Careful examination of a patient’s medical history and profile can lend clues to dysfunction of the salivary glands because they are often associated with other systemic disorders such as hormonal imbalances, diabetes mellitus, arteriosclerosis, and neurologic disorders. For example, both xerostomia, the symptom of dry mouth, and sialorrhea, increased salivary flow, could result from dysfunction of the medullary salivary center, autonomic innervation to the glands, damage to the gland itself, or imbalances in fluid and electrolytes.[24]
Consideration of drug history is also important, as xerostomia is a side effect of many medications, such as stimulants and antihypertensive drugs.[24]
Gender and age are also important variables. Sjogren syndrome (SS), an autoimmune disorder common in menopausal women, is another cause of xerostomia. Other symptoms of SS include dry mouth, dry eyes, dry skin, arthritis, and fatigue. Natural aging leads to more viscous saliva via reduced ptyalin and mucin production, and therefore changes saliva composition rather than quantity produced. Tumors of the salivary glands are not uncommon, and radiation therapy from head and neck cancer should be noted, as they often cause persistent xerostomia from damage to the acini.[24]
Sialogogic agents (sialogogues) such as pilocarpine and cevimeline are one effective management strategy for managing xerostomia. Pilocarpine non-selectively stimulates muscarinic receptors that enhance secretions of both the salivary and lacrimal glands. Other options include topical sialogogues and saliva substitutes, which aim to replace saliva and offer enhanced mucosal wettening.[25]
Sleep-related xerostomia refers to the sensation of dry throat and mouth leading to disruption of sleep for water intake.[26]
Pathologies
Technetium 99m pertechnetate (TPT) scans of the head and neck are a useful diagnostic tool with respect to the salivary glands. The rate of salivary flow, xerostomia, and sialectasis (cystic dilation of the ducts) are all associated with reductions in TPT uptake in the glands. These scans are also helpful for discriminating between different varieties of salivary tumors, as well as identifying the relative size and extent of mass lesions.[27]
Sialadenitis, or inflammation of the salivary gland, can be caused by infection, irradiation, allergies, and trauma. Common infections include mumps, a viral sialadenitis that occurs predominantly in children. Symptoms include pain in front of the ear, parotid swelling, chills, headaches, and fever. Moreover, reduced protection of the oral cavity to bacteria caused by xerostomia results in higher indices of gland infection due to ascending organisms.[28]
Sialolithiasis is a common condition referring to the formation of calcified salivary stones, or sialoliths, within the gland. They usually form in excretory ducts, the most common of which is Wharton’s duct, but are also seen less commonly in the parotid, sublingual and minor salivary glands. Blockage usually leads to pain, swelling, and inflammation of the gland, all of which are exacerbated upon increases in salivary flow. In more severe cases, symptoms include pus, infection, and bad breath. While the mechanism of stone formation is not well understood, it is thought to be a result of aberrant calcium metabolism, dehydration, changes in pH due to infections, and xerostomia.[29][30][31]
Traumatic injury can cause the formation of mucoceles, a tearing of the salivary gland resulting in spillage of mucins into the surrounding tissue. It manifests itself as a bluish bubble and typically appears on the lower lip. A mucocele of the sublingual gland is known as a ranula, and it occurs on the floor of the mouth. Ranulas typically are relatively larger than mucoceles in other areas of the oral cavity. In rare circumstances, they have been known to descend into the neck, and are called “plunging” ranulas in this case. Mostly, ranulas are asymptomatic, but if large enough must be excised.[32]
(Click Image to Enlarge)
(Click Image to Enlarge)

Diagram of an acinus, as well as the three main types of ducts through which salivary fluid is secreted. Zymogen granules of acinus are represented by white dots at apex of acinar cells. Red cells covering acini and intercalated ducts are myoepithelial cells. Blue cells of intercalated duct represent single layer of cuboidal cells. Columnar cells of striated duct with basolateral membrane invaginations are represented by purple cells. Pseudo-stratified columnar cells of excretory duct are represented by green cells.
Contributed by Mousa Ghannam