Congenital Melanocytic Nevi
- Article Author:
- Peter Macneal
- Article Editor:
- Bhupendra Patel
- Updated:
- 10/1/2020 10:41:31 PM
- For CME on this topic:
- Congenital Melanocytic Nevi CME
- PubMed Link:
- Congenital Melanocytic Nevi
Introduction
A congenital melanocytic nevus (CMN) is a skin lesion characterized by benign proliferations of nevomelanocytes and presents at birth or develops within the first few weeks.[1] These lesions may also be referred to as giant hairy nevi, the term conveying the frequent clinical presence of excess hair growth.
CMN are principally classified based on size into small, medium, and large or giant. This is determined by the projected adult size of the maximal diameter of the lesion. Small CMNs are projected to be <1.5cm in diameter, medium 1.5 to 19.9cm, and large or giant >20cm.[2] The scaling factor used to predict adult size is based on anatomical location. CMN located on the head are predicted to grow by a factor of 1.7, on the lower limb 3.3, and upper limb and torso by 2.8.[3] The rationale for classification based on size is due to larger lesions having a higher risk of melanoma, cosmetic implications, more challenging surgical excision, and higher rates of associated symptoms.[4]
Etiology
CMNs develop in utero from the 5th to the 24th week of gestation due to localized genetic abnormalities with resulting over-proliferation of melanocytes.[5] Melonoblasts, the precursor cells to melanocytes, begin migrating from the neural crest to the epidermis between the 8th and 10th gestational weeks.[6] A common CMN mutation is N-Ras. This is hypothesized to lead to the overproliferation of melanocyte lineage cells and thus the formation of CMNs.[7]
Dysfunction of the cytokine hepatocyte growth factor, which regulates melanocyte migration and proliferation, has also been implicated in this condition.[8] Although some evidence exists of familial etiology, the majority are considered to be caused by sporadic mutations.[6]
Epidemiology
Although the estimated prevalence varies between studies, smaller CMNs are considered to be relatively common lesions, while large CMNs are rare. The estimated incidence of small CMNs is 1 in 100 newborns, medium CMNs 1 in 1000, and large or giant CMNs between 1 in 20,000 to 1 in 500,000 newborns.[3][5][9] A slightly higher incidence in females than males has been reported with a 3 to 2 ratio.[6]
Histopathology
CMNs are typically characterized by nevomelanocytes or nevus cells in the epidermis in well-ordered clusters as well as in the dermis as cords, sheets, or nests. When compared to acquired nevi, CMNs more commonly extend deeper into the dermis or subcutaneous fat layer.[10]
The nevus cells cluster around dermal appendages such as the pilar apparatus, neurovascular structures, and sebaceous glands.[5][10] Nevus cells may also extend as cords of cells between bundles of collagen within the reticular dermis.[11]
History and Physical
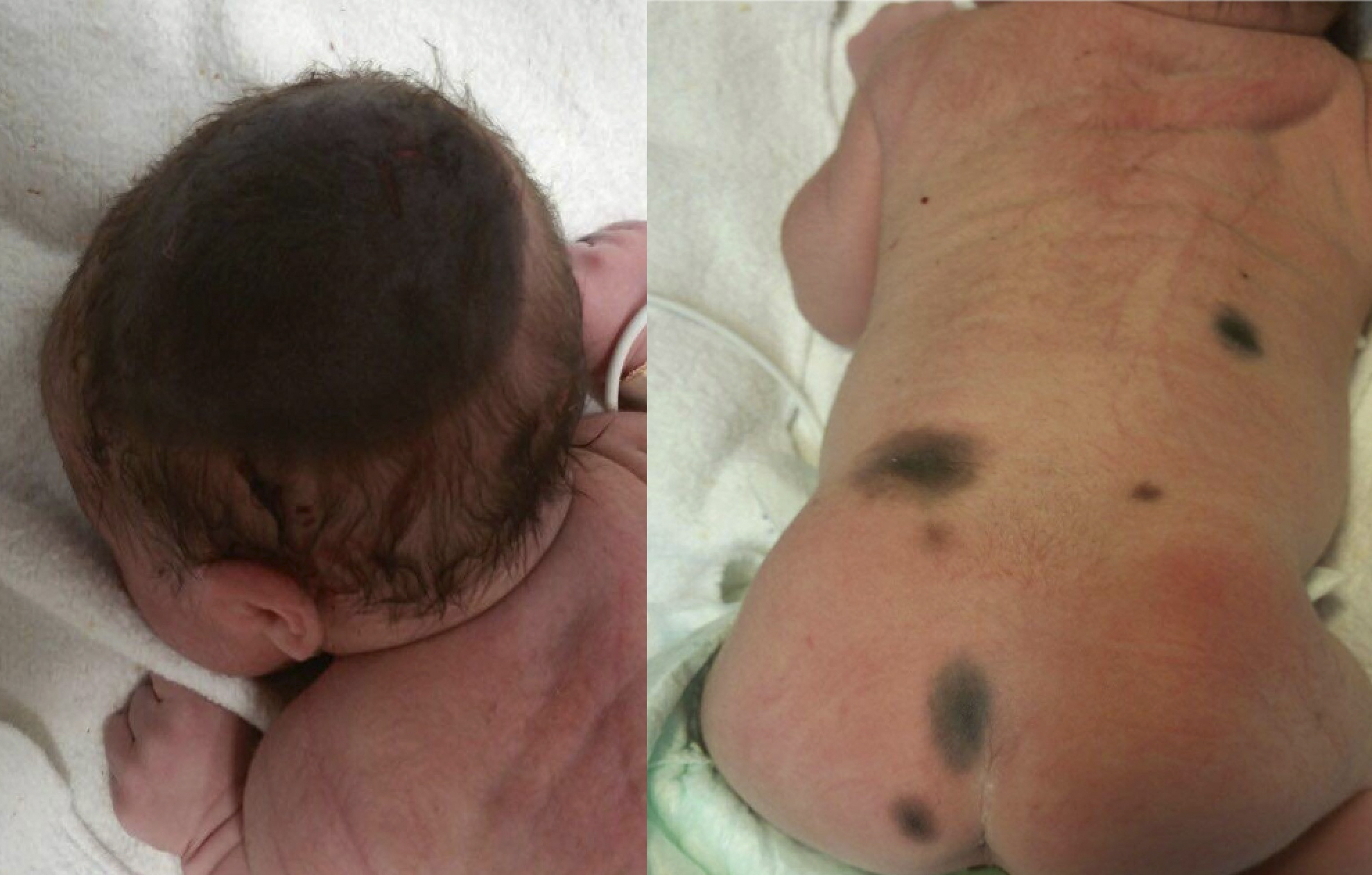
CMNs are usually noted at birth or in the neonatal period. CMNs of small to medium size normally present as well-defined round or ovoid lesions with a smooth surface and uniform brown color. While larger CMNs may present with an irregular border, different pigmentations, and rugous or nodular surface textures.[3] Satellite nevi are also more common in larger CMNs (see image). They occur in approximately 80% of giant CMNs.[11]
CMNs may evolve over time with changes in pigmentation, development of dermal nodules, and development of hair in some lesions. Pigmented hair growth or hypertrichosis has an incidence of 75%.[6] Nodule formation is more common in larger lesions and usually represents a benign proliferation of melanocytes.
Although usually asymptomatic, larger lesions may have clinical features of xerosis, ulceration, pruritis, or skin erosion.[12] Furthermore, the unsightly aesthetic appearance can cause significant psychosocial impacts on children and parents alike.[6]
A particular type of nevus is the "divided nevus." These are paired nevi that may occur on the penis, fingers, or eyelids. They are sometimes called "kissing nevi." Kissing nevi were first described by Fuchs in 1919. These nevi are a single lesion up to the 24th week of gestation. When the eyelids separate after the 24th week of gestation, the nevi divide. Although these nevi are generally present since birth, they can also present at a later age. Associated problems with eyelid kissing nevi are epiphora, eyelid malposition (ptosis or ectropion), or amblyopia.[13]
Evaluation
CMNs are usually diagnosed by their clinical appearance. Examination with dermatoscopy or biopsy for histology may be used in cases of diagnostic doubt.
Dermatoscopy evaluation most commonly reveals globular pigmentation patterns, while structureless, reticular, or mixed patterns may occur.[6] One study defined structures that differentiate CMNs from acquired nevi as the presence of small globules, vessels, follicles, and target networks.[14]
Tissue biopsy may be used as a means of ruling out malignant differential diagnoses through histopathological assessment.
Treatment / Management
Treatment options may be divided into surgical and non-surgical. Surgical options include tangential excision, curettage, en bloc or serial excision and direct closure, and excision with reconstruction using tissue expansion, skin graft, or flap.[15]
Non-surgical treatments include dermal abrasion, chemical peels, cryotherapy, electrosurgery, and ablative lasers.[5] These may be considered to reduce pigmentation and improve the cosmetic appearance of the nevus without fully removing nevi cells.
Historically the rationale for treatments for CMNs was influenced by improving the aesthetic appearance and reducing the risk of malignant change. However, excision surgery to reduce malignancy risk remains controversial. Advocates of surgery argue that the incidence of melanoma is lower in groups of patients who have undergone excision than those managed with observation.[16] Arguments against surgery include the low absolute incidence of melanoma in CMN balanced with surgical risks. Excision does not impact the risk of extracutaneous melanoma occurrence. Extracutaneous melanoma accounts for a significant proportion of new melanomas in this population.[2] As a result of this, many centers do not offer surgical excision on the basis of risk reduction alone. Instead, they offer surveillance, unless there are aesthetic or function indications for surgery. Further randomized studies are warranted.
Differential Diagnosis
- Acquired melanocytic nevus
- Congenital blue nevus
- Lentigo
- Mongolian spots
- Nevus spilus
- Becker nevus
- Café-au-lait spots
- Pigmented epidermal nevus
Prognosis
Small and medium CMNs pose lesser risks in terms of malignant transformation and neurocutaneous melanosis complications. These lesions generally have a good prognosis for patient survival. When melanomas arise in large or giant CMN, the prognosis is poor. Reported 5-year survival in 35 giant CMN patients with diagnosed melanomas was 34.3%.[4] This poor prognosis is hypothesized to be due to delayed clinical detection due to the deeper origin of cutaneous tumors, as well as de novo extracutaneous melanoma formation.[5] The deeper cutaneous origin of the melanomas may also cause accelerated lymphatic and metastatic spread, with one report indicating 24% of melanomas in patients with giant CMN had metastases at the time of diagnosis.[17]
For patients with symptomatic neurocutaneous melanosis, the prognosis is also unfavorable. One study reported 50% of patients dying within 3 years of symptomatic onset, with no curative treatments yet identified.[18]
Complications
Complications are more common in patients with larger CMNs, ranging from localized cutaneous irritation to malignant melanoma transformation.[11] CMNs may develop symptoms of cutaneous irritation such as ulceration and pruritus, as well as distortion in the structure and function of certain anatomical sites. A psychological burden to children and parents based on aesthetic appearance is a frequent sequela in such cases. With larger lesions, systemic involvement may comprise limb abnormalities and scoliosis.[19] The most severe complications include malignant melanoma and neurocutaneous melanosis.
Malignant melanomas may develop in any patient with CMN, although the risk is higher in patients with large lesions >20cm in size, or with multiple CMNs.[19] Malignant transformation may occur cutaneously, or extracutaneously, including the central nervous system, retroperitoneum, and the mucosa of the gastrointestinal tract. The reported absolute risk of melanoma with CMNs varies between studies. Patients with small to medium CMNs have an absolute risk of 0 to 4.9%, while in patients with large CMNs, the absolute risk is 1.25 to 10.00%.[3][20][21] Due to the higher risk, patients with large CMNs require long term monitoring for features of both cutaneous and extracutaneous malignancy.
Meningeal melanocytosis is a condition associated with giant or multiple CMNs, characterized by benign or malignant proliferation of melanocytes in the central nervous system.[5] Neurocutaneous melanosis may be asymptomatic or symptomatic regardless of malignant transformation. Although this condition has been estimated to affect 5%-10% of patients with giant CMN, the vast majority are likely asymptomatic.[3] The true incidence has not been determined. In neurocutaneous melanosis, the presence of melanocyte proliferation or hemorrhages may cause clinical features of raised intracranial pressure such as seizures, focal cranial nerve palsies, or hydrocephalus.[11] These diagnoses are made through magnetic resonance imaging (MRI) radiology and with medical and neurosurgical management within the interprofessional setting.
Deterrence and Patient Education
Knowledge of the appearance of CMNs by healthcare professionals is vital to identify these lesions at birth or soon afterward. It is important to ensure appropriate diagnosis and referral to dermatology. Due to the higher risk of severe complications, patients with large CMNs require long term monitoring for features of both cutaneous and extracutaneous malignancy.
Enhancing Healthcare Team Outcomes
An interprofessional team approach is strongly advocated in managing children with this condition, particularly for larger lesions. Pediatricians and family medicine clinicians should be able to diagnose CMNs and refer the patient to dermatology. Regular assessments involve dermatology examinations, with input from plastic surgery as required. Monitoring for progression with serial photographs and for malignant progression with input from pathologists for tissue diagnosis as required is also important.[11]
Pediatric psychologists and psychiatrists also play an integral role in managing the psychosocial burden.[19] Pediatric nursing and allied health professionals also play a pivotal role in management. Communication and coordinated care between members of the interprofessional team is important to ensure good patient outcomes. [Level 5]
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)
(Click Image to Enlarge)