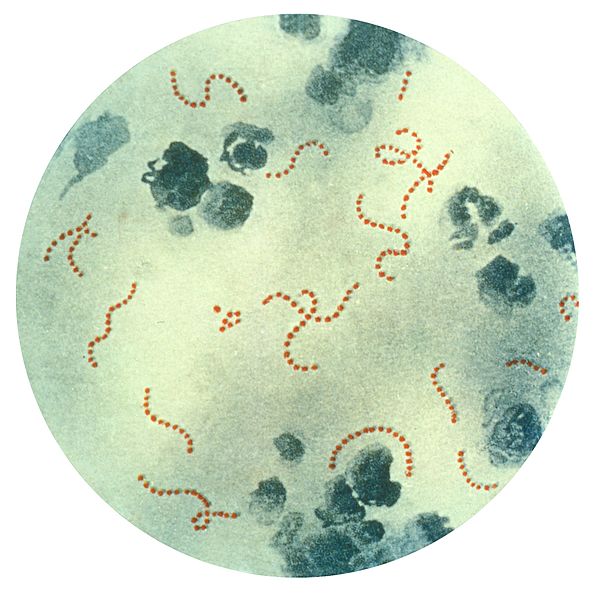
Streptococcus Group A
- Article Author:
- Ryan Newberger
- Article Editor:
- Vikas Gupta
- Updated:
- 10/5/2020 10:04:38 AM
- For CME on this topic:
- Streptococcus Group A CME
- PubMed Link:
- Streptococcus Group A
Introduction
Group A streptococci (GAS) are one of the most common bacteria encountered daily, and they result in acute infections with a wide array of manifestations in both adults and children and is responsible for an estimated 9,000-12,000 deaths per year in the United States.[1] Group A streptococci (GAS) are classified as gram-positive cocci that cause a range of diseases. GAS can be divided into greater than 100 different subtypes based on their surface ‘M-protein’. Infections due to GAS result in acute pharyngitis, impetigo, erysipelas, and cellulitis. GAS also tends to produce manifestations of more invasive diseases, including streptococcal toxic shock syndrome and necrotizing fasciitis. In addition to acute infection, infections due to GAS result in several nonsuppurative complications, including acute rheumatic fever and post-streptococcal glomerular nephritis.[2]
Etiology
Group A streptococci are ubiquitous bacteria that are found both as normal skin flora and in the environment around us. The different types of infections caused by GAS are produced in varying manners. For instance, GAS pharyngitis is caused by the transmission of bacteria from an infected individual through contact with oral secretions. Conversely, cellulitis secondary to GAS is caused by direct invasion of bacteria through breakdown/wounds in the skin, which can subsequently progress to necrotizing fasciitis. In a similar manner, impetigo and erysipelas are both caused by direct invasion through breaks in the skin’s protective barrier.
Epidemiology
The epidemiology of different infections secondary to Group A streptococci varies dependent on the type of infection. Pharyngitis secondary to GAS occurs primarily in children from 5 to 15 years of age. It can also be seen in adults but less frequently. Typically, pharyngitis secondary to GAS occurs most often in the winter and early spring. Overall, among patients with pharyngitis, about 15-30 percent of cases in children and 5 to 10 percent of cases in adults are caused by acute GAS infection.[3] Cellulitis and erysipelas are two other syndromes caused by various invasive bacteria, with approximately 10% of cases caused by GAS alone and a large number caused by mixed bacteria, including GAS.[4] Similar to cellulitis, impetigo is caused by several different bacteria, however, just under half of the cases are caused by infection with GAS.[5] Much less commonly found are the more invasive infections of toxic shock syndrome and necrotizing fasciitis. Toxic shock syndrome has been found in population-based surveillance data from Europe to have an incidence of about 3 cases per 100,000 individuals per year with no specific peak age or demographic for infection.[6] With regard to necrotizing fasciitis, population-based data from the United States has an estimated rate of 2.5 cases per 1,000,000 person-years.[7]
Pathophysiology
Recent advances in genetic techniques have allowed a more detailed analysis of the virulence of group A streptococci and how these factors function to promote infection with GAS. On the surface of GAS cells are found molecules of lipoteichoic acid and protein F, which help with adherence to host cells and successful colonization. GAS also produces chemicals called streptolysins and hyaluronidase, which function to destroy host tissues, thereby allowing GAS to spread through the host. Additionally, GAS has several factors, including its capsule, protein G, C5a peptidase as well as protein M, which function to protect GAS from host immune defenses. M protein, in particular, is thought to cause much of the virulence of GAS as it functions to inhibit phagocytosis by host immune cells.[8]
History and Physical
Given that group A streptococci have multiple typical associated infections, each infection type is typified by its own unique characteristics found during the initial history and physical.
For those experiencing pharyngitis secondary to GAS, the most common presenting features in the history are fever and sore throat, which are typically sudden in onset. Additionally, many patients complain of some combination of headache, nausea/vomiting, and abdominal pain. Also frequently found in history is close contact with an individual who is known to have a case of GAS. This is due to the fact that frequently there is a high prevalence in the community when new cases of GAS are being detected. Associated physical exam findings of GAS pharyngitis include generalized inflammation of the tonsils and pharynx with variable exudate. Also frequently found on the exam are tender cervical lymph nodes (lymphadenitis), red and swollen uvula, palatal petechiae, and sometimes a scarlatiniform rash. Very infrequently found are conjunctivitis, cough, coryza or diarrhea, and the presence of these points much more toward a viral cause of symptoms.[3]
Impetigo is a clinical diagnosis typically found in school-aged children. These children typically are well appearing on their initial presentation without acute signs of distress. They will present with cutaneous lesions that appear as discrete to confluent ‘honey-crusted’ lesions that typically appear on the face and/or extremities. Typically, no vital sign abnormalities are associated with the clinical presentation, and the patient will have no associated physical exam findings apart from the characteristic lesions.[5]
The more invasive GAS infection resulting in necrotizing fasciitis produces much more severe symptoms found on history and physical. Typically, these types of infections initially appear somewhat benign with an area of slightly reddened/inflamed soft tissue which can mimic simple cellulitis. However, one key finding early on is a disproportionately high amount of pain with the observed lesion. Following this, there is usually a rapid migration/spread of the erythema often greater than 1cm/hour despite treatment with IV antibiotics. Subsequently, blisters and bullae can form, which typically drain either serosanguineous or hemorrhagic fluid. The skin at this point will often turn necrotic and begin sloughing. Many times, crepitus will be found as subcutaneous gas is produced. Once further along in the clinical course, the pain will lessen significantly as the cutaneous nerves are destroyed by the infection. Throughout the course of necrotizing fasciitis, patients will experience fever, chills, and in more severe cases, will demonstrate hypotension and tachycardia typical of sepsis.[9]
Another serious and life-threatening condition caused by GAS is toxic shock syndrome. Toxic shock syndrome is caused by invasive infection by GAS, which results in bacterial enterotoxins being released, which results in systemic symptoms. These invasive infections can be the result of primary infection by GAS in a number of sites, especially deep wound infections. These patients clinically present with symptoms of severe sepsis with tachycardia, hypotension, poor tissue profusion, and signs of end-organ dysfunction.[6]
Evaluation
As discussed previously, several infections secondary to GAS are diagnosed based on history and physical alone, especially in the case of infections such as cellulitis, impetigo, and necrotizing fasciitis. Other infections, such as those causing toxic shock syndrome, can be suspected clinically, however, they are most effectively evaluated with blood cultures to identify the causative organism in order to tailor appropriate antibiotic therapy.
Pharyngitis secondary to GAS is one area where there has been a significant amount of recommendations regarding appropriate testing. The primary reason is that testing currently employed demonstrates a small amount of false negative as well as false-positive rates, which may result in inappropriate treatment or lack thereof if the testing is not used in an appropriate manner. Throat swab cultures are the gold standard for identifying cases of GAS pharyngitis. However, these results are not available for 24 hours or longer. Rapid swabs for GAS have a specificity of greater than 90 percent, but the sensitivity is still only between 80 to 90 percent. In children, the rate of infection is high enough (high pretest probability) that a positive rapid swab results in a high enough posttest probability that there is no need for a confirmatory throat culture to confirm the diagnosis of GAS pharyngitis. Conversely, in adults, given the low general rate of pharyngitis due to GAS (and therefore low pretest probability), it is actually a negative rapid swab that does not need to be confirmed by culture as a negative rapid swab confers an extremely low posttest probability of infection with GAS.[3][10]
Treatment / Management
Given that all infections due to GAS are bacterial in nature, the mainstay in treatment is targeted antibiotic therapy. In the majority of cases of infection due to GAS, antibiotic therapy can actually be started before confirmatory laboratory results are available. However, it logically follows that if testing is ultimately negative for GAS, antibiotics that have been specifically targeted against GAS should be discontinued.
When considering antibiotics for treatment of GAS, there are several options available. Penicillin based antibiotics, including ampicillin and amoxicillin, are very effective at the treatment of GAS infections. Given their similar mechanism of action, cephalosporins, in addition to macrolides and clindamycin, have shown appropriate activity against GAS.
Specifically, when treating GAS pharyngitis and simple cellulitis, typically, an oral regimen of the above antibiotics is recommended for a course of about 10 days in pharyngitis and 5-7 days in cellulitis. An alternate treatment specifically for pharyngitis due to GAS is a single dose of intramuscular penicillin G benzathine, especially in those patients who are unlikely to complete the full course of oral antibiotics.[3]
For more severe infection secondary to GAS, including necrotizing fasciitis and toxic shock syndrome, the causative bacteria is typically not known at the time of the patient’s presentation. Given the life-threatening nature of these infections, it is recommended that more broad-spectrum antibiotics be given in IV form. This ensures that both GAS, as well as other possible causative organisms, are treated while awaiting final speciation results.[6] In addition to antibiotics for these severe/systemic infections, supportive measures should be also taken, including fluid resuscitation, and blood pressure management with vasopressors, etc.[11][12]
Differential Diagnosis
As has been mentioned earlier, many bacterial and viral pathogens can cause identical presentations to those infections caused by GAS. As such, each infection must be evaluated with the previously mentioned bacterial cultures and/or swabs to evaluate for GAS versus other bacterial or viral infections. Additionally, there are several non-infectious conditions that can closely resemble GAS infections. Cellulitis, in particular, has a very similar presentation to both venous stasis changes as well as the same findings noted with an acute DVT, especially when symptoms are present in the lower extremities. Therefore, when evaluating a patient with lower extremity pain, redness, and/or swelling, it is important to keep these conditions in mind. In many cases, venous stasis changes are found bilaterally, which is much less commonly found in cellulitis, which could point toward the appropriate diagnosis. Conversely, unilateral redness, swelling, and/or pain could represent either an acute deep vein thrombosis or cellulitis therefore, ultrasonography is often needed to make the correct diagnosis.[9]
Prognosis
Generally, the prognosis for the less severe infections secondary to GAS is good with very low rates of morbidity and mortality. This is true of pharyngitis, cellulitis, and impetigo, which are effectively treated with standard oral antibiotic therapy and, in the vast majority of cases, even resolve spontaneously without medical intervention. Given the exceedingly low mortality rate from these types of infections, there is very little data to provide accurate quantification of annual mortality rates.
On the other hand, the more invasive and severe infections caused by GAS do carry with them a significant mortality and morbidity rate. Based on most recent studies, toxic shock syndrome itself carries approximately a 5-10% mortality rate with a worse prognosis for those at the extremes of age and those with preexisting conditions. Even worse, mortality rates are present in those diagnosed with necrotizing fasciitis as recent data have demonstrated mortality rates between 25% and 35% and a nearly ubiquitous need for surgical intervention, which adds to morbidity to those who survive their infection. An even worse prognosis exists for those who are concurrently diagnosed with toxic shock syndrome and necrotizing fasciitis as these individuals have mortality rates up to 60%.[6][10]
Complications
Apart from the morbidity and mortality associated with severe infections caused by GAS, the one major complication that can result from even minor infections is acute rheumatic fever. This is an autoimmune response typically to GAS pharyngitis. This leads to illness that is typified by joint pain and/or swelling, fever, chorea, skin and subcutaneous changes, and cardiac valvular regurgitation. The symptoms of acute rheumatic fever can be severe with significant edema due to heart failure, severe joint pain, high fevers, and choreiform movements that prevent the normal performance of activities of daily living.
This diagnosis typically requires management in the hospital for evaluation of the extent of symptoms with an echocardiogram, etc. The mainstay of treatment is first, the eradication of GAS from the body with antibiotics. Subsequently, in severe disease, corticosteroids or non-steroidal anti-inflammatory medications may be employed. In cases of chorea, antiepileptics may be needed, and aggressive management of heart failure symptoms may be necessary for those experiencing fluid overload due to valvular regurgitation. The valvular regurgitation associated with acute rheumatic fever may be permanent and, in some cases, is severe enough that it requires definitive surgical management with valve repair/replacement.[13] Another possible complication of GAS infection is post-streptococcal glomerular nephritis.
Deterrence and Patient Education
As with any infection, whether it be viral or bacterial, isolation and avoidance of exposure are important to avoid contracting the infection. This is especially true in cases of impetigo, and strep pharyngitis as these infections are much more common in children. As such, children have a higher propensity to spread this infection through close contact with other children. Therefore, it is important for isolating known infections from close contact with others by having them remain home from school, daycare, etc. However, due to the fact that GAS is ubiquitous in our environment and part of our normal skin flora, there is really very little that can be done to completely avoid infection with GAS.
Enhancing Healthcare Team Outcomes
One of the most important ways in which the interprofessional team can improve outcomes in patients with known or suspected infection with GAS is recognition of the signs of infection. This is especially important in cases of the more invasive/severe cases of GAS infection, such as those found with necrotizing fasciitis and toxic shock syndrome. This means that healthcare professionals must recognize signs of sepsis and shock and pay particular attention to physical exam findings, which would indicate deep soft tissue infections or systemic signs of infection. In doing this, the clinical outcomes among patients are greatly improved as appropriate antibiotics can be provided in a timely manner with the hope that the spread of the infection and the sequelae of the infection may be more promptly controlled. This requires appropriate coordination of nursing and clinicians to recognize these signs of infections, consultants to aid in achieving an appropriate diagnosis, and pharmacists to help treat these infections most effectively giving consideration to the clinical presentation and the local antibiotic resistance patterns.
(Click Image to Enlarge)