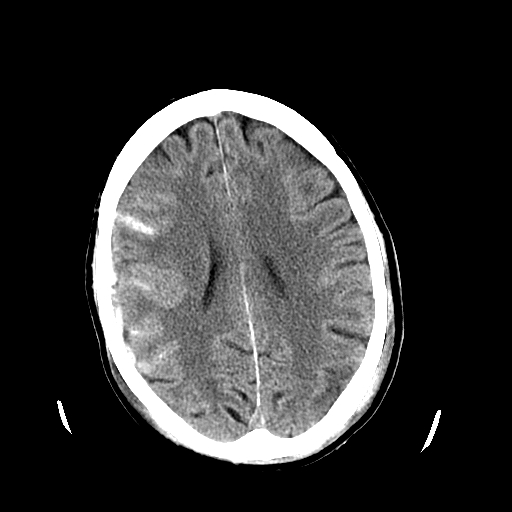
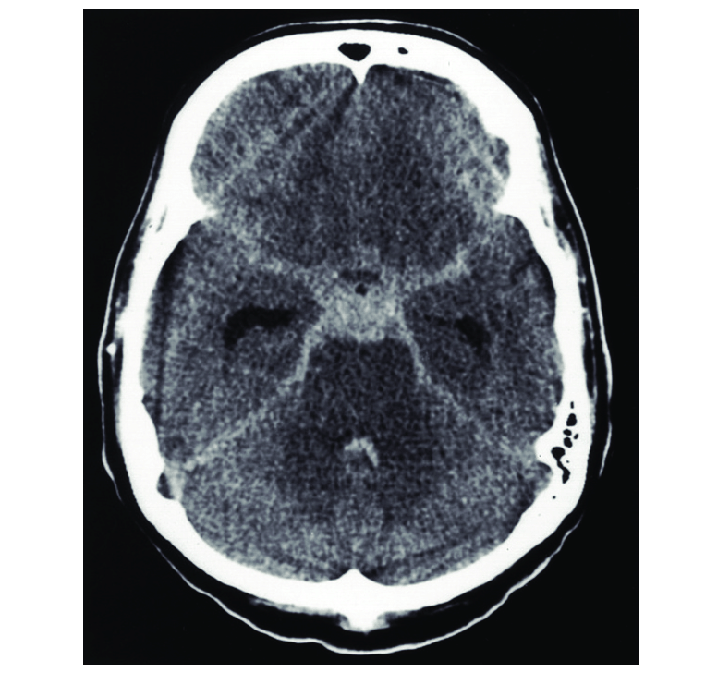
Acute Subarachnoid Hemorrhage
- Article Author:
- Norah Kairys
- Article Author:
- Joe M Das
- Article Editor:
- Manish Garg
- Updated:
- 10/13/2020 11:15:03 AM
- For CME on this topic:
- Acute Subarachnoid Hemorrhage CME
- PubMed Link:
- Acute Subarachnoid Hemorrhage
Introduction
A subarachnoid hemorrhage (SAH) results from medical aneurysmal rupture or traumatic head injury.[1] SAH occurs in the subarachnoid space between the arachnoid membrane and the pia mater that surrounds the brain.[1] Patients typically present complaining of a severe headache; however, only 10% of patients presenting to the emergency department complaining of a thunderclap headache end up having a SAH.[1] Associated symptoms may include neck pain, nausea/vomiting, and photophobia.[2] Other similarly presenting diseases include meningitis, migraines, acute narrow-angle closure glaucoma, cerebral venous sinus thrombosis, and non-SAH intracerebral hemorrhage.[2]
Etiology
Although head trauma causes some cases of SAH, up to 85% is the result of a ruptured saccular aneurysm.[3] These aneurysms often occur within the Circle of Willis and its branches. Other precipitators of a SAH include cocaine abuse, sickle cell anemia, anticoagulation disorders, and dissection of a vertebral artery.[3]
Anyone with a family history consisting of two or more first-degree relatives who have had an aneurysmal SAH will require preventative screening. Additionally, autosomal dominant polycystic kidney disease (ADPKD) is known to be associated with cerebral aneurysms in up to 8% of patients.[3] Therefore, patients with ADPKD also require screening if they have even one family member with a history of a ruptured aneurysm.[3]
Surgical correction of cerebral aneurysms was first introduced in the 1930s, and by the 1990s coiling, and clipping became popular less-invasive treatment options for patients with a SAH. The International Study of Unruptured Intracranial Aneurysms (ISUIA) has established prognostic data for patients with a history of a prior SAH or patients who were found to have an aneurysm as an incidental finding on brain imaging. They have concluded that aneurysms that are smaller than 10 mm or an aneurysm that has never bled are very unlikely to have a SAH and may not require surgical repair as a preventative measure.[4]
Epidemiology
Spontaneous SAH happens in about one per 10,000 people per year.[5] Females are more commonly affected than males.[5] Although this clinical entity is more common with older age, about 55% of patients with an SAH present under age 55.[5] Patients are at an increased risk of SAH if they have a history of smoking, hypertension, or excessive alcohol consumption.[6] Up to 10% of patients with a SAH report a history of bending over, lifting heavy objects, or performing other strenuous activities, at the onset of their symptoms.[5]
Pathophysiology
Saccular aneurysms account for most cases of SAH.[7] These intracranial aneurysms are thin-walled protrusions from an intracranial artery that often has a thin (or absent) tunica media as well as an absent internal elastic lamina.[7] Often hemodynamic stress leads to excessive wear on the wall of these arteries leading to turbulent blood flow within the vessel, which causes structural fatigue and aneurysm development.[7] Hypertension, cigarette smoking, and a variety of connective tissue diseases can also contribute to arterial wall breakdown.[6]
Fusiform aneurysms are caused by dilation of the entire circumference of the vessel, often formed as a result of atherosclerosis.[7] Mycotic (infected) aneurysms often result from emboli due to infective endocarditis.[7] However, these forms of aneurysms are less likely to rupture and cause an SAH than a saccular aneurysm.
Traumatic SAH usually occurs near the site of a skull fracture and intracerebral contusion. Radiologic clues of a traumatic origin include localized bleeding in a superficial sulcus, an adjacent skull fracture, and a cerebral contusion with external evidence of traumatic injury.[8]
History and Physical
Patients presenting with a SAH classically present with a “thunderclap” headache (characterized as a severe rapidly progressing headache that develops within seconds to minutes and has a maximal intensity at its onset), neck stiffness, vomiting, decreased level of consciousness, hemiparesis, and occasionally, seizures.[2] The typical headache pattern is described as a pulsatile pain that propagates towards the occiput.[2] Seizures are more common if an aneurysm causes the SAH, and a SAH in a patient with a history of seizures is often diagnostic of a cerebral arteriovenous malformation.[2]
Neck stiffness typically does not occur until about 6 hours after the onset of a SAH. Asymmetrical pupil size and loss of the pupillary light reflex may indicate brain herniation caused by rising intracranial pressure. Terson syndrome (vitreous hemorrhage as a result of a severe SAH) occurs in anywhere from 3% to 13% of cases.[2] Increased intracranial pressure can lead to a sympathetic surge due to activation of the descending sympathetic nervous system at the medulla, which causes a local release of inflammatory mediators that activate the sympathetic system in the peripheral circulation.[9] This sympathetic surge may lead to an increase in blood pressure, cardiac arrhythmias, and/or cardiac arrest.[9] Electrocardiographic changes may include large U waves, T wave abnormalities, QT prolongation, Q waves, high R waves, cardiac dysthymias, and ST changes (elevation or depression).[9] Neurogenic pulmonary edema may occur if fluid from the pulmonary capillaries leaks into the alveoli as a result of increased pressure within the pulmonary circulation.[9]
Patients may report a history of a head injury before the onset of symptoms or a known history of a cerebral aneurysm. Other risk factors include high blood pressure, smoking, family history, and drug/alcohol abuse.
Patients may also report having a history of a prior severe headache and/or a history of a small bleed with resolving symptoms within the past month. This is considered to be a sentinel bleed, which often precedes a more severe SAH. A headache from a sentinel bleed is extremely difficult to diagnose, as CT and LP can be unreliable in these cases.
Some physical exam findings include an oculomotor abnormality/palsy, which may indicate the posterior communicating artery as the source of the bleed.[2] The patient may demonstrate neck stiffness on examination if there is meningeal irritation from the SAH.[2] In this case, Kernig sign (inability to fully extend the knees when the thigh is flexed at the hip and knee is at 90-degree angles) and Brudinski sign (hip and knee flexion with passive neck flexion) may also be positive.[2]
Evaluation
Generally, if a non-contrast head CT is obtained within 6 hours of symptom onset, the diagnosis can be determined based on this imaging.[10] Almost 99% of cases are picked up on head CT if performed within this 6-hour window.[10] However, MRI is considered to be more sensitive than head CT as time goes on.[1][11]
If the non-contrast head CT is indeterminate, or if the patient presents outside of the 6-hour window, a lumbar puncture (LP) should be discussed with patients.[12] Lumbar puncture has been found in some studies to show evidence of hemorrhage in 3% of patients with a normal head CT.[12] Conversely, a recent study concluded that LP, in their cohort of neurologically intact CT-negative emergency department headache patients, did not identify any cases of aneurysmal SAH but was associated with serious complications, a significant false-positive rate, and extended emergency department length of stay.[12] Thus, attempts to investigate SAH (or sentinel bleeds from a presumed small aneurysmal leak) with CT scan and lumbar puncture should be discussed with patients in a shared decision-making approach with informed consent/refusal.
With regards to laboratory testing, consistently elevated red blood cell counts in all cerebrospinal fluid (CSF) tubes may indicate a SAH. The CSF can also be examined for xanthochromia (yellow appearance to the centrifugate fluid) by spectrophotometry or visual examination.[12] Additionally, the presence of bilirubin within the CSF may also indicate a SAH; however, it takes a minimum of 12 hours for hemoglobin to be metabolized so this method can only reliably detect a SAH 12 hours after its onset.[12]
Lastly, cerebral angiography or CT angiography can be used to identify an aneurysmal source of the bleed. [12]
In 1968, Hunt and Hess established a SAH severity scale based on symptoms at presentation.[13] The Fisher Grade was later created in 1980 to classify the appearance of the SAH based on the appearance of the CT scan.[13] In 1988, the World Federation of Neurosurgeons classification was developed to include the patient’s Glasgow coma score (GCS) and focal neurological deficits to gauge symptom severity.[13] In 1998, Ogilvy and Carter created a comprehensive classification system to help predict outcomes and gauge therapy for patients with a SAH.[14] Lastly, Claassen modified the Fisher Grade scale in 2001 to incorporate the additive risk from a concomitant intraventricular hemorrhage.[13]
Treatment / Management
The first step in the management of a SAH is to stabilize the patient and to get an emergent neurosurgical evaluation.[12] Patients presenting with a decreased GCS may require intubation for airway protection.[15] Blood pressure, pulse, respiratory rate, and GCS must frequently be monitored.[12] Pain control and antiemetics are often required for symptom control.[12]
Once the diagnosis of a SAH is made, most patients will be admitted to a neurosurgical intensive care unit as up to 15% of these patients may have worsening bleeding during their admission.[15][16][15] An external ventricular drain (EVD) may be indicated if the patient has a poor clinical grade on admission, acute neurological deterioration, or progressive ventricular enlargement on CT.[15] This EVD may be used to remove CSF or blood that can cause increased intracranial pressure.[15]
Patients with a large hematoma, decreased level of consciousness, or any focal neurological deficits may require surgical removal of the blood and/or occlusion of the bleeding site.[12] If a cerebral aneurysm is identified on angiography, clipping or coiling can be used to reduce the risk of further bleeding. Clipping requires a craniotomy to visualize and place clips around the neck of the aneurysm. Coiling is an endovascular technique that locates and deploys coils within the aneurysm from a catheter that is inserted into the femoral artery.[17] Aneurysms of the middle cerebral artery tend to be amenable to clipping, whereas those of the basilar artery and posterior cerebral artery are typically more accessible by endovascular coiling.[17] The International Subarachnoid Aneurysm Trial (ISAT) demonstrated a better prognosis from the endovascular coiling of the anterior cerebral artery and anterior communicating artery aneurysms than with clipping. However, coiling carries a slightly increased risk of aneurysm recurrence, so these patients are typically followed for several years with repeat angiography for monitoring purposes.[17]
Early predictors of rebleeding include high systolic blood pressure, the presence of hematoma within the brain or ventricles, poor Hunt-Hess grade, posterior circulation aneurysms, and any aneurysm greater than 10 mm in size.[18] Systolic blood pressure should be kept below 140 to 160 mm Hg to prevent rebleeding.[12] Labetalol is commonly used for this purpose.[12]
Calcium channel blockers such as nimodipine or nicardipine are often used to prevent vasospasm.[12] Vasospasm can lead to ischemic brain injury (delayed ischemia) as a result of the restricted blood flow caused by vessel constriction.[12] Delayed ischemia often presents with new neurological symptoms and is confirmed by transcranial Doppler or cerebral angiography.[12] A blood flow velocity of more than 120 centimeters per second on transcranial Doppler suggests vasospasm.[19] Up to one-third of patients with a SAH have vasospasm, and about half of these patients have permanent deficits as a result.[19] Nimodipine has been shown to improve patient outcomes if given between the fourth and twenty-first day after bleeding from an aneurysmal SAH. [19] However, nimodipine has not been shown to affect long-term outcomes in traumatic SAH and is therefore not recommended in these cases.
Differential Diagnosis
- Meningoencephalitis
- Cluster headache
- Adult seizures
- Intracranial hemorrhage
- Ischemic cerebrovascular accident
- Migraine
- Transient ischemic attack
Prognosis
Unfortunately, SAH is often associated with a poor outcome.[20] Nearly half of patients presenting with a SAH caused by an underlying aneurysm die within 30 days, and a third of those who survive have complications. Roughly half of the patients who have had a SAH suffer from some neurocognitive impairment that impacts their quality of life.[21] Over 60% report ongoing and recurring headaches.[21]
Complications
Cerebral vasospasm typically occurs after the third day of onset and typically reaches its peak on the fifth to the seventh day.[22][19] Blood products released from the SAH stimulate the tyrosine kinase pathway, which results in smooth muscle contraction of the cerebral arteries leading to vasospasm.[22] If vasospasm does occur, it can be treated with intravenous fluids to achieve a state of hypertension, hypervolemia, and hemodilution.[22] This triad is often referred to as “Triple H.”[22] However, to date, no randomized controlled trials have been conducted to support its utility. If vasospasm continues despite this medical management, angiography may be attempted to identify the site of spasms and administer intra-arterial vasodilator medication or angioplasty with balloon stenting.[22]
Other reported complications of SAH include hydrocephalus, hypopituitarism, cardiac decompensation, fluctuations in blood pressure and electrolyte levels, and seizures.[23][24] It has been reported that seizures occur in up to a third of SAH hospitalizations.[20][17] Although antiepileptic drugs are often given to prevent seizure occurrence, research has yet to show any benefit from its administration.[20] Some studies have suggested a worse prognosis and increased risk of gastric hemorrhage associated with these medications, but the etiology of these findings remains unclear.[20]
Enhancing Healthcare Team Outcomes
SAH is a severe life-threatening emergency that demands prompt treatment. The majority of patients present to the emergency department; thus, the emergency department team must know how to assess the patient and order the appropriate tests. Neurology and a neurosurgical consultation are mandatory. In addition, the intensivist and Neurology ICU nurses must be familiar with the management of these patients. Unfortunately, the disorder carries mortality over 50% irrespective of treatment. Even those who survive are left with severe complications that are disabling. Thus, an ethics team should be involved early on to discuss with the family the options of DNR.[25]