Syringomyelia
- Article Author:
- Varadaraya Satyanarayan Shenoy
- Article Editor:
- Raghuram Sampath
- Updated:
- 6/29/2020 11:47:09 PM
- For CME on this topic:
- Syringomyelia CME
- PubMed Link:
- Syringomyelia
Introduction
Syringomyelia, at its core, is a disorder of an abnormal cerebrospinal fluid (CSF) circulation. A syrinx is a fluid-filled cavity that anatomically lies within the spinal cord parenchyma or the central canal[1]. This entity is most frequently associated with a CM-I,[2] although other known causes include spinal cord tumor, trauma, and post-traumatic or infectious adhesive arachnoiditis.[3][4] Although syringomyelia predominantly presents with sensory symptoms such as pain and temperature insensitivity, in most cases it is an incidental finding.[1][5]. The discovery of syringomyelia is becoming more common because of the increased use of MRI in the routine evaluation of back and neck pain.[5]
The natural history of patients with syringomyelia is variable and unpredictable punctuated with periods of stability and progression. Although professionals do not completely understand the natural history of syringomyelia, the clinical course progresses over months to years, with an early rapid deterioration that gradually slows down.[6] It is also understood that sudden jolting of the head, a prolonged bout of coughing may bring a sudden onset of symptoms in a previously asymptomatic patient presumably because of an increase in tonsillar descent.[2][7]
Syringomyelia accounts for up to 5% of paraplegias.[8] The quality of life in syringomyelia patients is comparable with that of patients with heart failure or malignant neoplasms.[9]
Etiology
The etiology of syringomyelia includes conditions that alter the physiologic CSF circulation dynamics. In most cases, it is secondary to spinal subarachnoid space obstruction. Etiology includes
Idiopathic Syringomyelia
Syrinx in the absence of an identifiable cause
Secondary Syringomyelia
Syringomyelia with obstruction at the foramen magnum (developmental)
- Chiari 1 Malformation (CM1): Most common association[2]
- Basilar invagination
Syringomyelia with other diseases of the spinal cord (acquired)
- Post-inflammatory
- Post-infectious: Granulomatous (tuberculosis, fungal), post-operative meningitis
- Chemical/ Sterile inflammation: Post-subarachnoid hemorrhage (SAH), post-myelography (metrizamide)
- Post-traumatic
- Spinal Cord Tumors: Intra-medullary spinal tumors esp. Hemangioblastoma
- Secondary myelomalacia: Cord compression (herniated disc, spondylosis, tumors), infarction, hematomyelia
Epidemiology
Epidemiological data on syringomyelia is limited. Some studies have found the prevalence of syringomyelia to be from 8.4/100,000 to 0.9/10,000 with ethnic and geographic variation.[10][11] Majority of the patients present between ages 20 to 50. Familial aggregation, twin studies and known genetic syndromes associated with CM1 and syringomyelia together suggest a genetic component of transmission.[2][12]
Pathophysiology
Authors have proposed various theories to explain the origin and progression of syringomyelia.
- Gardner and Angel publicized the "hydrodynamic theory." Hindbrain hernias obstruct the normal CSF egress. Therefore, the fluid swerves into the central spinal canal pulsating into the central spinal canal with each heartbeat ("water-hammer" effect).[13]
- Williams found pressure gradients of over 100 mg Hg across foramen magnum during Valsalva maneuvers in hindbrain hernia patients and postulated that this cranial spatial pressure differential creates a suctional force ("suck") that pulls the ventricular CSF into the syrinx. The fluid drawn into the cavity would then impact-and-bounce off ("slosh") the caudal and subsequently rostral walls of the syrinx,[14][15] similar to the behavior of water in an elastic balloon squeezed at one end. This theory was no longer in vogue after subsequent authors underscored the lack of communication between syrinx and the fourth ventricle.[16][17] However, recently this theory has found evidentiary support on dynamic MRI studies. Slow caudal growth of a syrinx may follow 42 million such impacts during a year.[18]
- Ball and Dayan argued that CSF drives through the spinal cord surface along para-vascular Virchow-robin spaces because of increased spinal CSF pressure (coughing, sneezing) in patients with foramen magnum obstruction.[19]
- However, there are no compelling large-scale physiological studies to refute or confirm any of these three proposals.[20]
- Oldfield et al. proposed that a CSF pressure wave generated because of systolic "piston-like" caudal displacement of tonsils, contracted the cord forcing fluid into the syrinx cavity from the spinal subarachnoid space.[16] However, a critique is that fluid cannot enter a contracted cord. While studies have been able to show a downward movement of the tonsils during systole on dynamic MRI, there has been no visual confirmation of trans-parenchymal CSF movement on CINE MRI.[18]
- Gretiz[1] and Koyanagi and Houkinkoya[21] have proposed a theory supporting extracellular nature of syrinx fluid rather than CSF. Abnormally rapid movement of CSF past the cord causes a low-pressure zone within the cord, promoting extracellular fluid build-up and syrinx formation.[18]
Although the pathophysiology concerning the origin of syringomyelia remains an elusive Gordian knot, the pathophysiology underpinning progressive syrinx enlargement may have a common theme.[18]
Clinicopathological Correlation
The basic pathology in syringomyelia is a progressively expanding cavity in the central spinal canal. This expanding CSF filled "syrinx" compresses the spinothalamic tract neurons decussating in the anterior white commissure. However, the posterior columns are spared as they are located distally. This results in loss of pain and temperature sensation with preserved touch and vibratory sense (segmental dissociated sensory loss). The upper limbs are preferentially involved in a "cape-like" distribution (sensory loss predominantly in the shoulder area).
History and Physical
Since syringomyelia is commonly associated with Chiari malformation type 1 (CM1), it is pertinent to look at clinical features directly related to Chiari Malformation 1.
- Tussive headaches: Classic of CM1. Coughing (Valsalva maneuvers) causes sudden increases in intracranial CSF volume in CM1 patients which presents as transient suboccipital headache and neck pain, a split second after a cough.[2] It is important to understand the features of Chiari headaches to prevent misdiagnoses
- Site: Sub-occipital
- Mode of onset: Sudden
- Nature/character: Heavy, crushing, pressure-like. Pounding in quality when severe but otherwise non-throbbing[2]
- Radiation: Radiates to the vertex and behind the eyes, and inferiorly to the neck and shoulders
- Duration: Short duration-often, lasting only seconds
- Exacerbated by physical exertion, Valsalva maneuvers, head dependency, sudden changes in posture
- Hoarseness, dysphagia, coughing with swallowing because of downward traction on the lower cranial nerves and brainstem compression.
- Visual disturbances: Retro-orbital pressure and pain, visual phenomena such as flashing lights, floaters, blurred vision, photophobia, diplopia; possible horizontal, rotatory or down-beat nystagmus on clinical examination because of the involvement of brainstem vestibulo-ocular connections
- Oto-neurologic symptoms: Dizziness, tinnitus, pressure in ears, decreased hearing, oscillopsia
- Cerebellar disturbances such as tremors, dysmetria, ataxia, gait and balance problems.
- Syncope[22]
- Sleep disturbances such as snoring, sleep apnea and palpitations because of brainstem compression
Features caused by the syrinx will depend on its anatomical level and include:
- The most common sensory symptom is paresthesia/hyperesthesia followed by non-radicular segmental pain.[2] Loss of pain and temperature sensation with preserved touch and vibratory sense (segmental dissociated sensory loss). The upper limbs are preferentially involved in a "cape-like" distribution. Due to the lack of pain perception, patients often develop injuries, chronic skin ulcerations on the hand.
- Muscle weakness, impaired fine-motor function which may progress to atrophy of intrinsic muscles of hand – damage to the anterior horn cells (lower motor neuron) at the level of the syrinx. May progress to "claw hand" deformity.
- Spasticity in lower limbs: Syrinx expansion may compress and sometimes destruct adjacent lateral corticospinal tracts (upper motor neuron).
- Progressive scoliosis: Injury to anterior horn cells innervating the paraspinal axial musculature[23]
- Horner syndrome may be seen in cervical/upper thoracic syringes
- Poorly localized non-radicular segmental neuropathic pain (substance P)[24]
The presentation is highly variable. In most cases, patients complain of pain, muscle weakness and atrophy especially in hands and arms, temperature insensitivity in the upper limb, spasticity or stiffness in lower limbs, progressive scoliosis. The clinical course progresses over months to years, with an early rapid deterioration that gradually slows.[6] There is a linear relationship between the cyst morphology, symptom duration, and severity.[25][26] If there is associated Chiari Malformation 1, patients may also complain of an occipital headache (precipitated by coughing, straining, sneezing, among others), neck pain, gait, and balance problems, dizziness, hoarseness, problems in swallowing, sleep disturbances such as snoring.
In patients with syringomyelia secondary to foramen magnum obstruction treated only a syringosubarachnoid or a thecoperitoneal shunt, it has been observed that the spasticity improves despite the worsening of other neurological functions. This suggests the spasticity in the limbs is possibly due to the bulk of the syrinx rather than the compression of the brainstem at the level of the foramen magnum.[27]
Evaluation
MRI
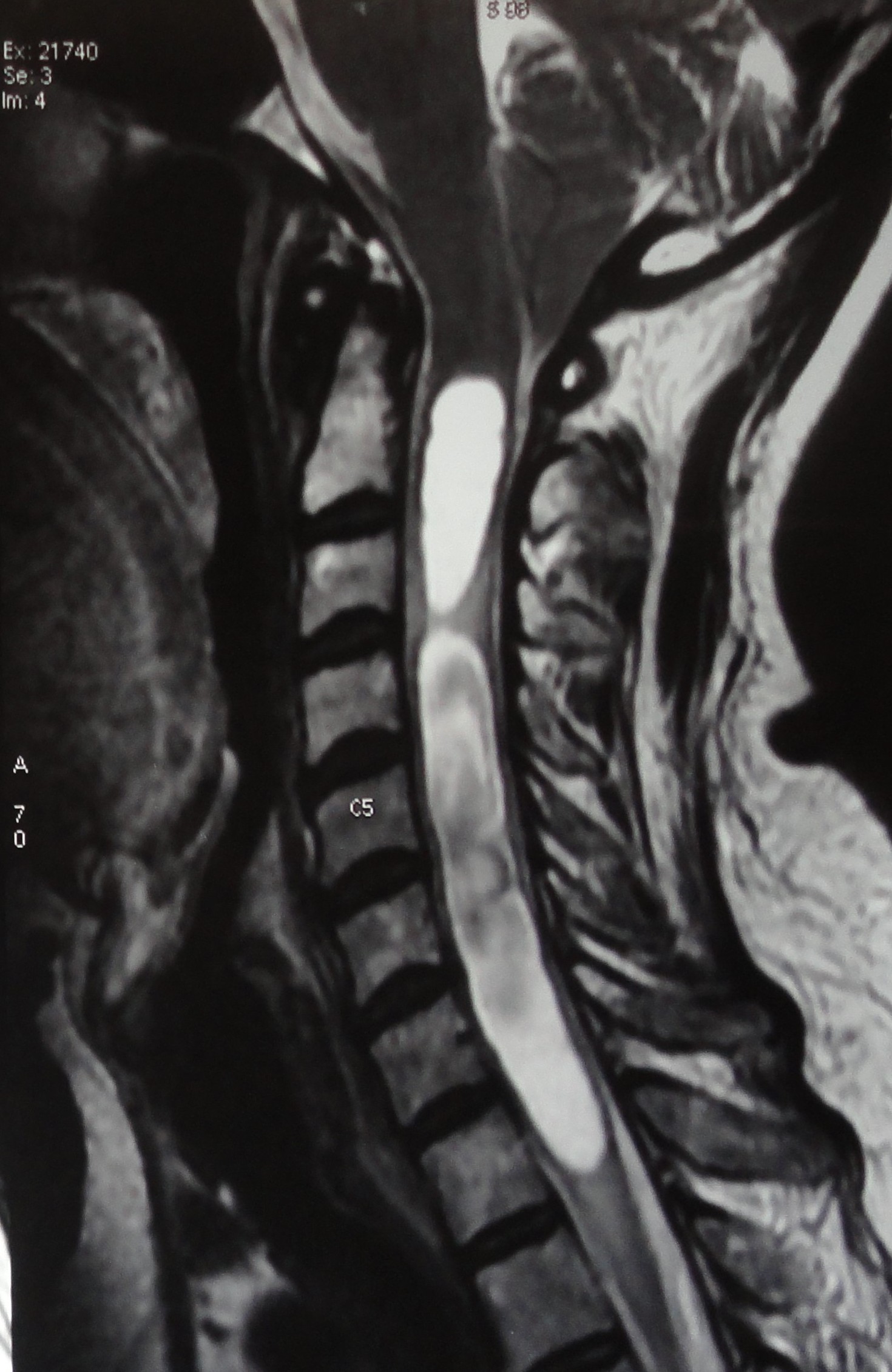
MRI with and without contrast is the investigation of choice. It delineates relevant anatomy and allows accurate visualization of the syrinx in both sagittal and axial planes. MRI easily reveals the location, size, and extent of the syrinx cavity, the degree of cerebellar tonsillar ectopia. A ubiquitous feature in patients with associated CM1 is compression of retro-cerebellar CSF spaces. MRI also helps to rule out cystic lesions or spinal tumors. Leptomeningeal enhancement indicates infection. MRI can also reveal any arachnoid scarring. One can also study syrinx progression over months or years to document the natural history of syringomyelia.
Dynamic MRI or Cardiac Gated CINE-MRI Flow Study
These can analyze CSF hydrodynamics non-invasively. It can diagnose CSF velocity/flow disturbance at foramen magnum (esp. in patients with <5mm tonsillar ectopia), visualize spinal cord wall motion and syrinx fluid motion at rest during the cardiac systole and diastole.[2][18] It is also useful to document postoperative CSF flow changes and objective improvements.
Myelography with High-Resolution CT Scan
This scan is indicated where MRI cannot be used (patients with metallic implants in the joints, cardiac pacemaker). Delayed CT scans can visualize dye leeched into the syrinx cavity. However, some authors have criticized CT myelography for having a low sensitivity in detecting CSF blockage.[28]
Electromyography has no diagnostic value in syringomyelia, but it helps to rule out peripheral neuropathy causing paresthesias.
Treatment / Management
The goal of treatment is to correct the underlying causative pathophysiology. All current treatment strategies are directed toward improving CSF flow dynamics.
In patients with Chiari malformation 1, craniocervical decompression is the best way forward.[3] This consists of suboccipital craniectomy and removal of the posterior arch of C1, opening the dura and arachnoid, and resection of arachnoid adherences when present.[29] What this surgery essentially does is that it creates an artificially enlarged cisterna magna. Intraoperative ultrasonography can be employed to confirm the decompression of the tonsil and pulsatile flow of the cerebrospinal fluid around the craniovertebral junction. The duration of sensory deficits best predicts the symptomatic improvement following surgery. Studies have shown that shorter duration of the preoperative symptoms has better outcomes. Early surgery minimizes deficits.[30]
In the patients with post-inflammatory arachnoid scarring and post-traumatic syringomyelia, the operative procedure is directed toward reconstituting spinal subarachnoid CSF flow by arachnoid scar membrane resection, microsurgical lysis of arachnoid adherences and dural reconstruction.[30][31]
Shunts are indicated for idiopathic syringomyelia and patients that have not responded to other treatment. Most commonly used is syringosubarachnoid shunt (SSAS). If this fails syringoperitoneal shunt (SPS) may be used. While there are studies that claim to have better outcomes with SSAS as compared to foramen magnum decompression,[32] this thought is not universally echoed.[26][33] In most cases, shunts are not favored and are used as a last resort because of high complication and failure rates, and inability to resolve the underlying etiology.
Differential Diagnosis
- Spinal intra-medullary tumors such as hemangioblastoma, ependymoma, gliomas: Tumors may secrete exudative fluid (high in protein content) causing micro-cysts that can eventually coalesce. Most of these intramedullary tumors will enhance on contrast MRI (a syrinx does not enhance on contrast MRI). However, a true syrinx can occur within a tumor)
- Spinal intra-medullary cysts
- Myelomalacia
- Arachnoid cysts
- Glio-ependymal cysts
- Residual central spinal canal: The central canal of the spinal cord involutes with age. A persistent central canal is NOT an anomaly.
Prognosis
Professionals still do not understand the natural history of syringomyelia; it is unpredictable and highly variable which makes its prognostication very difficult. Although prognosis depends on the etiology, the degree of neurological deficit and the site and size of the syrinx cavity, syrinx diameter of more than 5 mm and associated edema predict a rapid deterioration. The rarity of the condition, variable natural history, and short follow-up make treatment results assessment difficult.[34] However, early surgery minimizes deficits and has better outcomes.
Complications
Myelopathy is the major complications because of the disease process itself. This can further lead to spasticity which may progress to paraplegia/quadriplegia, decubitus ulcers, recurrent pneumonia, and bowel and bladder dysfunction.[35]
Neurological complications following surgery include cerebrospinal fluid (CSF) leaks, infection, hemorrhage, recurrence of the syrinx.
Deterrence and Patient Education
- Patients with untreated syringomyelia should avoid straining during bowel movements (especially in the initial weeks after surgery, when they may be on narcotic pain medication which often causes constipation) coughing, sneezing which can worsen symptoms and further enlarge the syrinx. Patients must consult the doctor when symptomatic for a cough or constipation
- Similarly, patients with syringomyelia should avoid lifting heavy weights.
- Experts advise patients with Chiari malformation to avoid activities that exert significant strain to the neck such as roller coasters, trampolines. Patients should avoid contact sports such as football. They may practice alternative forms of exercise such as swimming, stationary bicycling, and yoga.
- The patient must immediately report any post-operatively drainage of purulent fluid from the surgical site or signs of infection, such as a painful, edematous, reddened wound, to the treating physician.
Pearls and Other Issues
- Syringomyelia(SM) should be considered high in the differential diagnosis in any young person with progressive scoliosis. Early identification and treatment can cause a reversal of deformity.[36]
- Hand involvement in syringomyelia patients is often asymmetric as syrinx cavity itself is eccentric in most cases.
- It is important for clinicians to distinguish Chiari headaches from other similar appearing headaches. History taking skills play a crucial role.
- History of a long duration of a headache and pain relief by neck support can help in identifying headaches originating in neck muscles that may extend into the suboccipital area.
- Posterior fossa brain tumors may produce similar headaches and also cause tonsillar descent. However, these headaches are of prolonged duration and have associated projectile vomiting. Fundoscopy in these patients may also reveal papilledema.
- Headaches in fibromyalgia patients may also mimic Chiari headaches.[37]
- Being a chronic disorder, a patient with syringomyelia not uncommonly has symptoms of anxiety, memory impairment, depression. The clinician is obligated to assess all symptoms with the right perspective, particularly the possibility of a specific symptom being mitigated by surgery. The wide availability of symptom lists on the internet, some which are non-specific adds to the challenge of eliciting an uncontaminated history from the patient.[38]
Challenges/Pitfalls
Identifying and distinguishing the root cause of deficits in post-traumatic syringomyelia, the syrinx cavity or cord injury itself, remains a challenge.
Enhancing Healthcare Team Outcomes
Post-operative pain is a major issue for patients and is often managed with opioids and muscle relaxants. It is important that the patient and his/her family understand that their physicians cannot overmedicate. Pain medications are a double-edged sword. The risk/benefit ratio must be weighed carefully. Oversedation with opioids may put the patient at risk for developing complications such as pneumonia. The physician must carefully titrate the dose of pain medications to allow maximum comfort with minimum risk of complications for the patient. It is crucial that the patient, family, and friends comprehend and respect this balancing act. A well-informed patient can assist their physician in the process by comprehending the proposed strategy for the diagnosis, workup and subsequent treatment.