Urea Breath Test
- Article Author:
- Senthilkumar Sankararaman
- Article Editor:
- Leila Moosavi
- Updated:
- 8/11/2020 11:48:57 PM
- For CME on this topic:
- Urea Breath Test CME
- PubMed Link:
- Urea Breath Test
Introduction
Helicobacter pylori (H. pylori) is a ubiquitous, microaerophilic, gram-negative, bacillus.[1][2] It affects more than 50% of the population worldwide and is one of the widely prevalent common chronic bacterial infections.[3][1][2] Drs. Barry Marshall and Robin Warren first isolated H. pylori in the year 1983.[4] The prevalence of H. pylori is variable between 19 and 88% and depends on various factors such as geographical location, patient's age, sanitation, and socioeconomic status.[3][1][5] Patients often acquire H. pylori infections during childhood, and if untreated, persist throughout life.[1][5][6]
H. pylori have strong associations with many non-neoplastic (peptic ulcer disease, chronic atrophic gastritis) and neoplastic (adenocarcinoma of the stomach, gastric lymphoma arising from mucosa-associated lymphoid tissue or MALT) conditions of the gastrointestinal (GI) tract.[7][8][9][2][5] Due to this strong association with malignant conditions, the World Health Organization (WHO) categorized H. pylori as a class I (definite) carcinogen in 1994.[10] Early diagnosis of H. pylori and successful eradication cures chronic gastritis and can reduce the progression to the long-term complications.[8] H. pylori gastritis is also implicated in non-ulcer dyspepsia.[11][8] Many hematologic disorders such as unexplained iron deficiency anemia and immune thrombocytopenic purpura in adults also have links with H. pylori, but the exact pathogenic mechanisms are unclear.[12]
Tests for H pylori include both invasive and non-invasive methods.[9] Each method has its advantages and limitations.[2] The selection of a test or combination of tests depends on many factors such as the clinical contexts, cost of the testing, availability, and their sensitivities and specificities.[9][2][13] The gold standard investigation for confirmation of H. pylori is esophagogastroduodenoscopy (EGD) with histopathological examination of the biopsy.[14][6] Further testing methods such as immunohistochemical staining, rapid urease test, bacterial culture and, PCR increase the diagnostic yield of EGD.[15][14] EGD also offers the advantage of evaluation of the long-term complications associated with H. pylori.[2] EGD is recommended in the evaluation of dyspepsia in patients over 60 years of life and also in the presence of alarm symptoms such as significant weight loss, gastrointestinal bleeding, abdominal mass, iron deficiency anemia and difficulty in swallowing.[8][16] However, EGD is invasive and expensive, limiting its utility as the first investigation of choice in young (less than 60 years of life) patients with dyspepsia and without alarm symptoms.[8][17][2][11] Also, an EGD evaluation may be falsely negative in patients who have a patchy distribution of the disease.[2][6][15] The popular non-invasive methods include urea breath test (UBT), H. pylori stool antigen test (SAT), and serological tests.
Reliable non-invasive methods include UBT and SAT.[13] UBT is the most accurate testing among the non-invasive tests.[18] SAT is cheaper but slightly less accurate than the UBT.[19][9] SAT involves stool collection, which may not be preferred by some patients.[8] H. pylori IgG serology is not recommended in places with lower prevalence rates of less than 30%.[19][8] With the lower prevalence of H. pylori, the pretest probability of diagnosing a true infection is lower.[20][19] Also, the antibodies against H. pylori persist for an indefinite period and does not differentiate past from present infections.[9][20][13] For the same reason, serological methods are not useful for evaluation of eradication after completion of treatment.[8][1] However, serological testing for H. pylori is useful for epidemiological studies and for screening larger populations in places with a higher prevalence rate.[13][2] Antigen-specific serological tests in whole blood, saliva are not recommended due to their lower predictive value.[19][8]
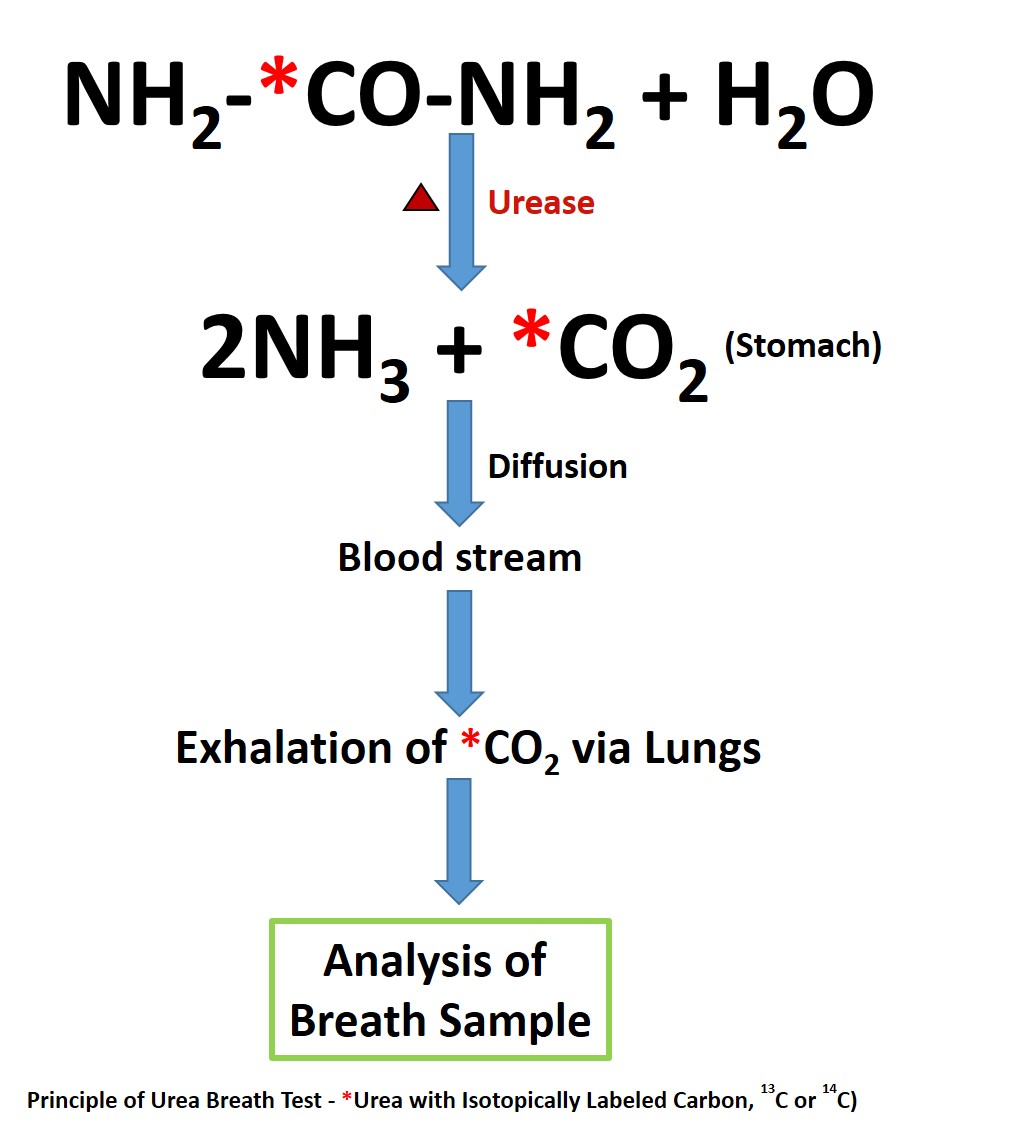
UBT is useful for both the initial diagnosis of H. pylori (test-and-treat strategy) and also in the evaluation of post-treatment status.[18] Urease is not present in mammalian cells, and hence its presence indicates urease containing microbes. H. pylori utilize urease to breakdown urea into ammonia and carbon dioxide (CO2).[15] The ammonia is utilized by H. pylori in the neutralization of gastric acidity and helps in its colonization.[13] Bacteria other than H. pylori are usually not present in the stomach with few exceptions such as in patients with achlorhydria.[21][22] UBT exploits this ability of urease, which is abundantly present in H. pylori to promptly hydrolyze urea into ammonia and carbon dioxide (CO2).[23] This CO2 enters the bloodstream and is later exhaled via the lungs. By using urea as a substrate with a labeled carbon (C) isotope, the exhaled CO2 with the labeled C, can be measured and quantified for the diagnosis of H. pylori. Testing commonly uses two isotopes of carbon for UBT - 14C (a radioactive isotope) and 13C (naturally occurring, stable non-radioactive isotope). The performance of both UBTs is almost similar, with sensitivities above 95% and specificities above 93%.[20][24][25]
Procedures
Before the UBT testing, patients should discontinue antibiotics, and bismuth compounds for at least four weeks and proton pump inhibitors (PPIs) and sucralfate for at least two weeks.[7][8][23][2] These medications can reduce the urease activity of H. pylori and can generate a false negative result.[26] In these scenarios, if the testing result is positive, the inference is that it is truly positive and treatment can begin. If the result is negative, then these medications should be discontinued as recommended above, and repeat the UBT.[7] Histamine 2-receptor antagonists should be ideally discontinued 24 to 48 hours before the testing to avoid a false negative result.[8][20][27] Use of antacids is permitted and do not seem to interfere with the UBT results.[20] Also following H. pylori treatment, UBT should be performed to evaluate eradication only four weeks after the completion of the treatment.[8]
14C-UBT: The commercial brand available for 14C-UBT in the U.S is PYtest. Current testing dose exposes patients to a small dose of 1 microcurie, which is equivalent to natural environmental background radiation received in about 24 hours.[23] The labeled urea with 14C is in the form of an oral capsule, which the patient takes after a period of fasting for 6 hours. Lukewarm water is used to swallow. The capsule should not be opened or crushed in the mouth to avoid contamination with oral flora, which may have urease activity. Ten minutes after the capsule ingestion, the patient is instructed to blow through a straw, and the breath sample gets collected in a balloon. Measurement of the labeled 14CO2 in the collected sample is by scintillation technique. In the presence of H. pylori, an increase in 14CO2 is noted due to hydrolysis of 14C-urea by the urease. In the absence of H. pylori, there will be no hydrolysis of 14C-urea. The unhydrolyzed 14C-urea is absorbed in the stomach, enters the bloodstream and excreted by the kidneys. Appropriate safety precautions are necessary for the storage, handling, and disposal of the radioactive test ingredients.[23] The overall cost of 14C-UBT is cheaper when compared to 13C-UBT.[8][23] Hence 14C-UBT is more popular in resource-limited settings.[2]
13C-UBT: Even though 14C-UBT is safe, the current preference is for 13C isotope as it is non-radioactive, especially in children, women of childbearing age and during pregnancy.[28][6][2][23][20] Graham et al. first developed the 13C-UBT.[29] In the past, gas-isotype ratio mass spectrometer was utilized to measure the labeled 13-CO2.[29][13][23] This method is costly and also needs technical expertise.[13][23] The newer methods utilize easier and relatively cheaper techniques such as infrared spectrophotometry or laser-assisted ratio analysis for analyzing the labeled 13CO2.[9][13][23] It is easily performed in clinic settings and also repeatable. 13C-UBTs can be administered in children as young as three years of age. Since 13C is natural and present everywhere, a baseline breath sample of 13CO2 is noted before the administration of labeled 13C-urea, and the baseline value is expressed as a ratio of 13CO2/12CO2. Unlike in 14C-UBT, a test meal is required here to delay the gastric emptying, and citric acid is the most commonly used test meal given with 13C-urea.[9][23] This citric acid reduces the duodenal pH, which in turn decreases the antral motility and also relaxes the fundus of the stomach.[23] 13C-urea with the citric acid mixture is ingested using a straw to minimize contamination with oral bacteria with urease activity. Fifteen minutes later, breath is collected again for analysis. An increase in the 13CO2 value from the baseline indicates urease activity, a surrogate for the presence of H. pylori. A post-urea ratio of 13CO2/12CO2 is noted. The difference in the ratios between the post-urea and the baseline is referred to as delta over baseline (DOB). There is no agreed consensus on the cut-off value for DOB to differentiate between H. pylori positive and negative results.[2] The threshold values for positivity varies widely with the DOB ranging from 2.7 to 7, depend on many variables such as sociodemographic disparities, patient characteristics (age, gender), bacterial factors, and laboratory specifications.[30][31] In the absence of urease, both the baseline and post-urea sample 13CO2/12CO2 ratios will essentially be the same. Unlike 14C isotope, another advantage of using 13C isotope is the breath samples can be sent safely from the clinics by mail to the laboratory for breath analysis.[23]
Indications
The urea breath test is useful for both initial diagnosis and eradication of infection.[9][23] Most patients with H. pylori are asymptomatic.[1] In the absence of alarm symptoms for gastric malignancy, UBT could be utilized to diagnose H. pylori.[23] Noninvasive tests, either UBT or SAT, are preferred for patients who do not need an endoscopic evaluation but have diseases strongly associated with H. pylori such as patients with a history of peptic ulcer disease (PUD), either with a history of active PUD or past medical history of PUD with no documented eradication.[5][19] For patients less than 60 years with dyspepsia, a non-invasive H. pylori test could be offered in the absence of alarming symptoms (GI bleeding, anemia, significant weight loss, loss of appetite, dysphagia, significant emesis, family history of GI malignancy, and history of GI cancer) and if the test result is positive, H. pylori treatment could be offered.[20][16][5][19][32] For patients over 60 years with dyspepsia, an EGD is recommended to evaluate for both H. pylori and more importantly, to evaluate also other causes such as malignancy.[16] Similarly, EGD could also be considered for younger patients less than 60 years of age who have a higher risk of malignancy such as a family history of gastric cancer or living in places with a high prevalence of gastric cancer.[16] Furthermore, H. pylori infections are also associated with other conditions such as unexplained iron-deficiency anemia (after a thorough negative evaluation), and immune thrombocytopenia in adults and testing for H. pylori can be a consideration.[5][19] Before the initiation or continuation of long-term NSAIDs or aspirin, the clinician needs to rule out H. pylori due to increased chances of development of PUD and its complications. [5] For eradication testing, UBT should be offered after 4 to 8 weeks of completion of the eradication treatment.[8] Here PPI should be discontinued at least two weeks before the testing to avoid a false negative result.[5][7][8]
Interfering Factors
False positive test results:
1. Helicobacter heilmannii also has urease activity and can produce positive results in UBT.[33]
2. Patients with achlorhydria can also have false-positive UBT results.[21] The acid environment usually inhibits the growth of bacteria other than H pylori. In patients with achlorhydria, other urease producing bacteria such as Proteus mirabilis, Citrobacter freunii, and Staphylococcus aureus has been reported to cause false positive UBT.[21][22][15]
3. Some of the oral flora have urease activity, and contamination with oral flora could also result in false positive results.[34] This contamination can be prevented or minimized by swallowing the 14C-urea in a gelatin capsule. Similarly, in the 13C-UBT method, the test meal and 13C-urea is recommended to ingest using a straw.
False negative test results:
1. Recent use of medications which interfere with H. pylori such as antibiotics, bismuth compounds, and, PPIs.[26] As mentioned earlier, patients should discontinue antibiotics and bismuth compounds for at least four weeks and proton pump inhibitors (PPIs) and sucralfate for at least two weeks before the testing.[7][8]
2. Similarly, following the H. pylori eradication, the UBT should be done at least four weeks after the completion of treatment. If done earlier, the result could be falsely negative.[8]
3. In patients with H. pylori infection predominantly in the body (corpus) of the stomach, data shows a higher proportion of false negative results with 13C-UBT.[35]
Clinical Significance
Advantages of UBT:
- Noninvasive, safer, and cost-effective when compared to endoscopic evaluation.[20] In patients with a patchy distribution of H. pylori, EGD evaluation could be negative. UBT has less tendency for sampling errors and more sensitive than EGD in these circumstances.[23]
- UBT is convenient and preferred by many patients when compared to stool collection for SAT.[20]
- UBT is more accurate than the SAT and serological tests.[1]
- UBT is helpful for initial diagnosis (test-and-treat strategy) and also to confirm the eradication of infection.
- 13C-UBT can be easily repeated and also could be administered safely in children (above three years of age), and in pregnant women.[23]
- 13C-UBTs are useful in the epidemiological evaluation of H. pylori in both children and adults.[9] Researchers also utilize serological tests in epidemiological studies of H. pylori and but unlike UBT, serological tests cannot differentiate past and present infections.[9]
Limitations of UBT:
- Helicobacter heilmannii, another urease containing bacteria can also cause false positive testing.[2]
- False positive test results in achlorhydria and also in oral contamination of bacteria with urease activity.[9]
- Recent history of upper GI bleeding could affect the accuracy of UBT.[1][2]
- 13C-UBT is less accurate in the pediatric population with sensitivity and specificity in the range of 75 to 100%.[25][9]
- Low sensitivity in patients with a history of gastric surgery.[15][2] EGD with rapid urease test and histology are preferable in this context.[2]