
Radiation Therapy For Heterotopic Ossification Prophylaxis
- Article Author:
- Anna Lee
- Article Author:
- Elizabeth Maani
- Article Editor:
- Neha Amin
- Updated:
- 10/7/2020 5:24:07 PM
- For CME on this topic:
- Radiation Therapy For Heterotopic Ossification Prophylaxis CME
- PubMed Link:
- Radiation Therapy For Heterotopic Ossification Prophylaxis
Introduction
Heterotopic ossification (HO) is defined as the formation of mature, lamellar bone in soft tissues where bone does not normally exist. HO is commonly seen following trauma or surgical intervention in periarticular soft tissue and is commonly associated with injury to the hip. The three primary causes can be grouped into traumatic, neurogenic and genetic etiologies.
HO from trauma includes fractures, dislocations, and operative procedures such as open reduction-internal fixation (ORIF) or total hip arthroplasties (THA). The compartment of the hip most involved is the abductor compartment [1]. The elbow is the next most commonly involved joint following burns or elbow replacements.
Neurogenic causes include spinal trauma and head injuries and again occur around major joints. The relationship between neurohumoral factors and the development of HO is poorly understood but is centered around stimulation of osteoblasts to lay down ectopic bone [2].
The last main etiology includes genetic disorders, such as fibrodysplasia ossificans progressiva, progressive osseous heteroplasia, and Albright hereditary osteodystrophy. These are rare conditions that lead to the development of HO early on in life, resulting in debilitating morbidity [3].
Etiology
The sensitivity of osteogenic progenitors to radiation therapy was known starting in the 1950s during experiments with bone repair [4]. These early studies harnessed the process whereby HO occurs; primitive mesenchymal cells in the surrounding soft tissues are transformed into osteoblastic tissue. This tissue then forms mature lamellar bone from a dysfunctional bone formation/remodeling process. It is thought that Craven et al. found osteogenic progenitors cells to be radiosensitive because of a high mitotic rate as they proliferate and differentiate into osteoblasts and chondrocytes [5]. Thus, low-dose radiation is used as a prophylactic treatment to decrease the chance of HO formation as discussed below.
Epidemiology
The incidence of heterotrophic ossification is more than 50% and as high as 90% in patients with moderate or severe osteophytes at the femoral head and socket in the setting of a hip replacement [6][7]. The hip is the most common site, followed by the elbow. Other major joints are sometimes affected. Soft tissue locations that are not associated with joints are rarely involved.
Several risk factors have been identified. High-risk patients include those with bilateral hypertrophic osteoarthritis, prior history of HO, and those with arthritis caused by trauma characterized by hypertrophic osteophytosis [8]. Moderate risk patients include those with unilateral hypertrophic osteoarthritis, ankylosing spondylitis, Paget disease, or diffuse idiopathic skeletal hyperostosis (DISH). Furthermore, HO is twice as common in males compared to females, age over 65 years at the time of surgery [9], and lateral surgical approach of the hip [10].
History and Physical
Most commonly, patients present with hip stiffness after surgery or trauma that can lead to decreased range of motion and functional impairment. Symptoms associated with severe HO may include fever, warmth, erythema, tenderness, and swelling. Pain alone within several days after surgery could be a presenting symptom of HO, but this presentation is less common.
HO also could be an incidental finding on radiograph where calcified structures with blurred contours are seen as early as two to six weeks postoperatively. Bone scans usually show increased uptake in the soft tissues adjacent to the hip. Scans can detect HO several days before it becomes apparent on plain film, but these findings are not specific for HO [11]. Maturation of the HO may take up to one or two years.
Rarely, correlations have been reported with sedimentation rate greater than 35 mm/hr [12] and a serum alkaline phosphatase greater than 250 international units/L in cases with severe HO [13].
Evaluation
HO is typically evaluated radiographically. The Brooker Staging system is the most widely accepted classification system and includes four grades based on anteroposterior (AP) radiograph of the pelvis and hip [14]. Grades 3 and 4 are considered clinically relevant, even if there is no pain or impaired mobility.
Brooker Staging System [3]
- Grade 1: Bone islands in the soft tissue around the hip
- Grade 2: Exophytes in the pelvis or proximal end of the femur with at least 1 cm between opposing bone surfaces
- Grade 3: Exophytes in the pelvis or proximal end of the femur with less than 1 cm between opposing bone surfaces
- Grade 4: Bony ankylosis between proximal femur and pelvis
Treatment / Management
Treatment depends on the severity and symptoms of HO or the risk that patient may have of developing HO. The orthopedic surgeon often will determine the type of HO prophylaxis the patient obtains. Early-grade cases that are asymptomatic may be observed. Advanced HO may require surgical resection followed by prophylactic modalities, such as medical therapy and radiation therapy (RT), to help minimize the high risk for re-development of HO. When a patient does not have a history of HO, the decision to offer prophylactic treatment is often weighed with patient-related, clinical, and surgical risk factors against the potential risks of preventative treatment.
Medical Therapy
The main medical therapy used for HO prophylaxis is nonsteroidal anti-inflammatory drugs (NSAIDs). While indomethacin is the most commonly used NSAID, other NSAIDs, such as ibuprofen, have been effective with the added benefit of ease of administration and low cost. The recommended dose of indomethacin is 75 to 100 mg/day for 7 to 14 days postoperatively, with care for gastrointestinal side effects such as gastritis and ulcer formation [15].
Differential Diagnosis
- Cellulitis
- Fracture
- Hematoma
- Local trauma
- Septic arthritis
- Thrombophlebitis
Radiation Oncology
Radiation therapy has been found to be more effective than NSAIDs in multiple trials. The effectiveness of indomethacin versus radiation therapy for HO prevention was compared in a prospective randomized trial comparing post-open reduction/internal fixation surgery for patients with an acetabular fracture. RT was given as 8 Gy in 1 fraction within 72 hours of surgery, and indomethacin was given for six weeks. There was a greater risk of nonunion of long bone fractures among those who received indomethacin over RT (26% versus 7%, p = 0.004) [16]. A subsequent meta-analysis by Pakos et al. analyzed seven randomized trials for a total of 1,143 patients and found that RT was almost twice as effective as NSAIDs but the absolute benefit was less than 2% [17] with efficacy being dose-dependent.
Radiation Dosing and Fractionation
External beam radiation therapy for HO prophylaxis is often prescribed to a dose of 7 to 8 Gy single fraction, but various studies have shown that several fractionation and dosing regimens are acceptable. Historically, 20 Gy in 10 fractions was administered based on pediatric observational studies of bony growth inhibition. Sylvester et al. retrospectively compared this regimen to 10 Gy in 5 fractions in 27 hips after THA and found the two groups to be similarly effective [18]. Around this time, Lo et al. retrospectively examined outcomes after single fraction RT with 7 Gy dose and found that none of the patients developed grade 3 or 4 HO [19]. Another single fraction regimen with 8 Gy was compared in a randomized trial with 10 Gy in 5 fractions in 62 hips. With a median follow up of six months, the failure rate in both groups was equivalent at 21% [20]. As the single fraction was shown to be effective, the efficacy of lowering the dose was explored by Healy et al. In this retrospective analysis, 107 hips were given either 7 Gy or 5.5 Gy postoperatively. The radiographic failure rate was 10% versus 63% in the lower-dose arm, which was significantly different [21]. A more recent study compared lower dose in a randomized prospective trial in 59 patients considered to be at high risk for HO. The two arms were 5 Gy in 2 fractions or 10 Gy in 5 fractions, and all patients were treated within four days of surgery. There was a trend toward increased HO of any grade in the 5 Gy arm (69% versus 43%, p = 0.09) [22].
Radiation Timing
RT is typically given either preoperatively within 24 hours or postoperatively within 72 hours. If there is a planned surgery, it is ideal for the patient to be consulted and consented prior to surgery since the anesthesia may deem the patient unable to give informed consent. Also, the patient could be in more pain post-operatively and may not be able to lie still during treatment. However, in trauma or emergency situations, the radiation would be given post-operatively.
A randomized, multi-institutional trial by Gregoritch et al. compared patients treated with pre-op versus post-op 7-8 Gy in 1 fraction and found that the radiographic and clinical failure rate between the two groups were not significantly different [23]. This was examined in another randomized study of 161 patients treated either preoperatively less than four hours before surgery or postoperatively less than 72 hours after surgery. There was not a significant difference amongst patients who had Brooker grades 0 to 2 however, treatment failures in the overall cohort were significantly lower in the postoperative group. This was likely due to higher biologic equivalent dose as the preoperative group received 7 Gy in 1 fraction, while the postoperative group received 17.5 Gy in 5 fractions. Despite these findings, the current standard is to use the same dose of single fraction 7 to 8 Gy within 24 hours pre-operatively or within 72 hours postoperatively.
Radiation Treatment Planning
The general principle of treatment planning should include standard techniques with immobilization devices if necessary and placement of the patient in a comfortable position that will allow reproducibility for daily treatments. The target volumes should include the surfaces of the bone that are most often involved with HO or the periarticular HO. HO often forms between the femoral head and pelvis, either from the greater trochanter to the ilium and/or the lesser trochanter to the ischium, so these surfaces must be included in the radiation field.
The radiation techniques to treat a hip with HO have been well described for the hip as this is the most commonly treated site. The patient is treated in the supine position in a comfortable and reproducible position. Each field is customized to the patient’s anatomy, but generally the cranial border is about 3 cm above the acetabulum, the inferior border about 3 cm below the lesser trochanter of the femoral head or could include the upper 1/3 of the implant shaft (if used) with a field size of about 14 x 10 cm. Soft tissue can be blocked by a combination of collimating the field to use the field edges and/or using the multi-leaf collimators to shape the field to allow about a 1.5 to 2 cm margin on the bone and prior areas of HO. Three-dimensional conformal radiotherapy (3DCRT) using anterior-posterior/posterior-anterior (AP/PA) fields with 6 to 15MV photons are often used to create a homogeneous plan to cover the target to at least the 95% isodose line while minimizing dose to the surrounding soft tissue and skin [Image].
Other Sites
The hip is the most common site of HO development and treatment. However, other joints are also susceptible to HO. RT has been effective in these areas as well, particularly with the elbow. A study of 36 patients treated at the Cleveland Clinic with 7 Gy in 1 fraction following elbow surgery found that RT was associated with favorable functional and radiographic outcomes [24]. Furthermore, a retrospective study by Strauss et al. of 44 patients found radiographic evidence of HO in 48% of the group with no complications after high-risk elbow surgery [25]. Favorable outcomes were reported from a case series of nine patients with clinically significant HO at the elbow. Fractionation included 5 Gy in 2 fractions and 6-7 Gy in 1 fraction with a median follow up of 7.7 months; there were no failures, with the majority showing clinical improvement [26].
Toxicity from Radiation
HO is not a malignant condition, yet it can cause pain, limited mobility, and negatively affect the quality of life. Thus the benefit of treatment must be weighed against the risks. Patients are often counseled that the low RT dose administered for HO prophylaxis decreases the risk of HO significantly with minimal side effects and is overall considered a safe treatment. Potential side effects are rare but include fatigue, wound healing delays, joint swelling, and an extremely low chance of secondary cancer from the RT.
Secondary malignancies from radiation can develop in the bone or tissues that were included in a previous RT field. Since the total dose that is given for HO prophylaxis is very low, the changes of secondary malignancies are also extremely low, but not zero. There have been at least two case reports describing secondary malignancies after single fraction. The first case described a 51-year-old patient who received 7 Gy x 1 for HO 15 years earlier. He underwent a second course of 7 Gy x 1 for debilitating HO but developed high-grade undifferentiated sarcoma of the proximal thigh 16 months after his second treatment [27]. Another case described a 26-year-old male who underwent surgery for an acetabular fraction and received 7 Gy in 1 fraction postoperatively for HO prophylaxis. He presented 11 years later with osteosarcoma of the treated area, which was treated definitively with neoadjuvant cisplatin and adriamycin with plans for surgical resection at the time of the report [28]. Otherwise, there are essentially no other reported cases of secondary malignancies from RT given for HO prophylaxis treatment as it is a very rare complication.
Trochanteric nonunion and wound healing complications are also potential toxicities from RT. One study found that the rate of nonunion ranged from 12% to 30% after RT, while the rate of nonunion was diminished in 2% to 15% of the time for those who did not get RT [3]. Since modern hardware for total hip arthroplasties is cementless and porous, they may rely on bony ingrowth at the acetabulum or the proximal femur region. There is concern that RT may inhibit the necessary bony ingrowth and lead to prosthesis failure, which has prompted some providers to utilize shielding of the acetabular cup to prevent this complication. However, a clinical study of patients receiving preoperative and postoperative radiation found no evidence of prosthesis failure, even without shielding [29].
For male patients, the RT dose to the testicles is of concern as there can be a reduction in sperm count, even from doses as low as 20 Gy. Use of testicular shielding is recommended as it can reduce dose to the testicles by approximately 50%.
Pearls and Other Issues
- Heterotopic ossification (HO) is the abnormal formation of bone in soft tissues where bone does not normally exist.
- HO is most commonly seen following trauma or surgical intervention in periarticular soft tissue and is commonly associated with injury to the hip.
- The use of radiation to the peri-articular joint prescribed to 7 to 8 Gy in 1 fraction given less than 24 hours preoperatively or less than 72 hours postoperatively can decrease the risk of HO development.
- Prophylactic RT for HO is safe and has been shown to be more efficacious compared to NSAIDs.
Enhancing Healthcare Team Outcomes
HO is best managed by an interprofessional team including orthopedic nurses. Today radiation is being recommended in select patients and while effective, one has to weigh the benefits versus harm of this therapy in asymptomatic patients. The patient should be involved in the decision-making process because RT is not entirely harmless.
(Click Image to Enlarge)
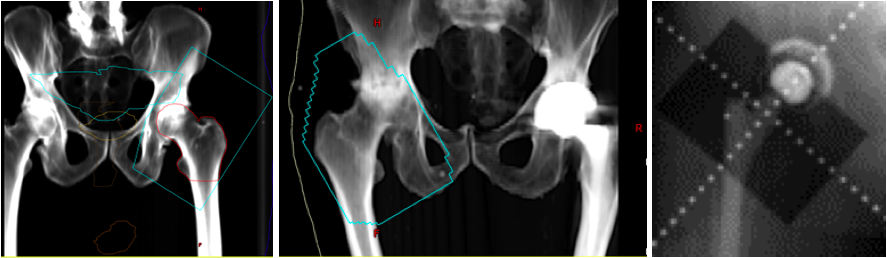
Digitally reconstructed radiographs (DRR) based on original portal images showing fields of three patients receiving radiation for heterotopic ossification of the hip. The blue line represents the field edge in the first two images. Take note that the surfaces of the greater trochanter to ilium and the lesser trochanter to the ischium are not blocked out since this is the most common location for HO formation. The third image shows shielding of the acetabular cup to prevent bony ingrowth and prosthesis failure.
Contributed by Neha P Amin