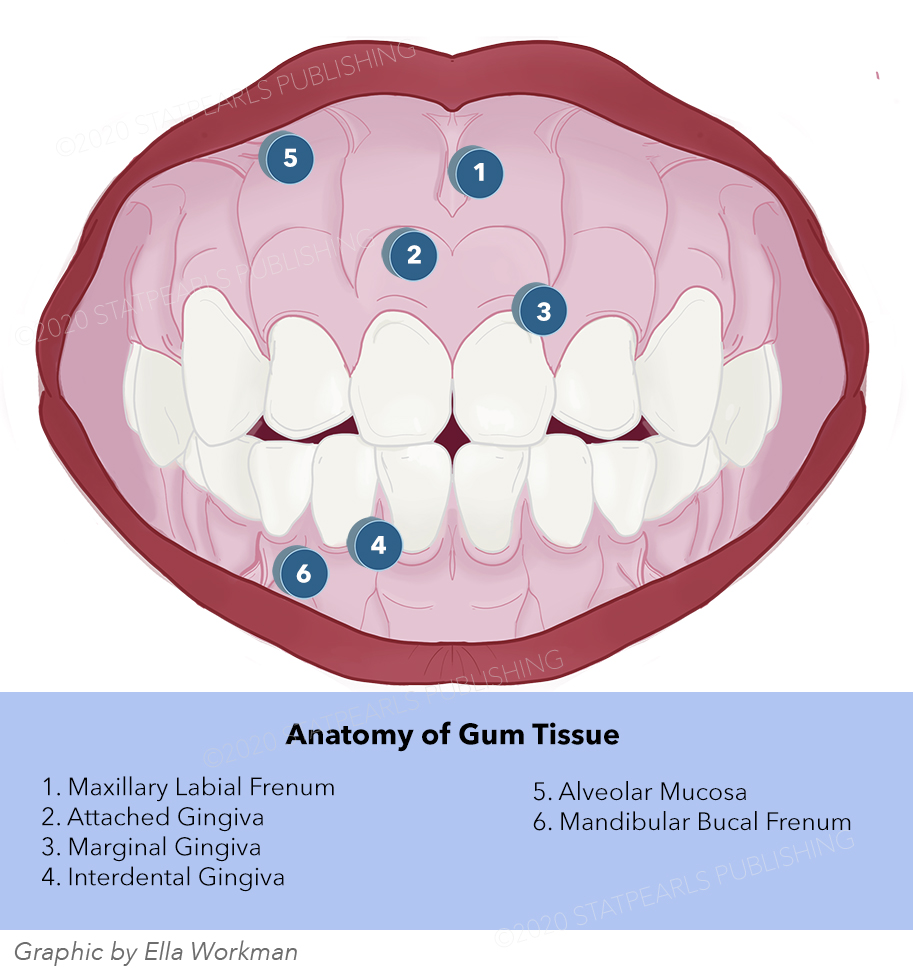
Anatomy, Head and Neck, Oral Gingiva
- Article Author:
- Adam Koller
- Article Editor:
- Amit Sapra
- Updated:
- 8/19/2020 1:42:06 PM
- For CME on this topic:
- Anatomy, Head and Neck, Oral Gingiva CME
- PubMed Link:
- Anatomy, Head and Neck, Oral Gingiva
Introduction
The gingiva, also known as the gums, is the pink-colored keratinized mucosa that surrounds and protects the teeth. It is perfused by multiple small arteries that originate from branches coming off of the carotid artery. It receives innervation by nerves derived from the mandibular and maxillary divisions of the trigeminal nerve. The gingiva is part of the periodontium, which includes the investing and supporting structures of the teeth. Additionally, signs of systemic disease frequently manifest in the mouth, and poor oral gingival health can worsen many systemic conditions. This article provides a comprehensive review of the anatomical features and embryological origins of the gingiva. Physiological variants in the gingiva and key surgical and clinical considerations are also covered.[1]
Structure and Function
The periodontium is responsible for supporting and maintaining healthy teeth. It is comprised of four components: the gingiva, periodontal ligament, alveolar bone, and cementum. The gingiva, also known as the gums, is a specialized epithelial tissue that surrounds the teeth via specialized cells known as junctional epithelial (JE) cells. This junctional epithelium is strategically located at the bottom of the gingival sulcus, where it acts as a barrier to both mechanical trauma and microbiological insult. In addition to its protective function, the oral gingiva is also responsible for sensation in the mouth and absorption of micronutrients. Lastly, the gingival epithelium plays a crucial role in the innate immune response to infectious inflammation in periodontal tissue. Thus, it is a key mediator in the initiation of periodontal disease.[2]
The gingival connective tissue (also known as the lamina propria) is composed of collagen fibers, cells, and ground substance. Cells make up 5% of the gingival connective tissue, while collagen fibers and ground substance make up 60% and 35%, respectively. The different cell types present in gingival connective tissue include fibroblasts, mast cells, macrophages, and inflammatory cells. Fibroblasts are the predominant cell type and are responsible for the formation of the collagen fibers and ground substance found in connective tissue.[3]
Collagen plays a significant role in the structural integrity of the gingival connective tissue. The basic unit of collagen is tropocollagen, a triple helix made up of 3 polypeptide chains. Collagen forms through a series of steps, which include translation, hydroxylation, glycosylation, and peptide cleavage. Once tropocollagen is formed, these molecules aggregate intro fibrils via crosslinks between each staggered tropocollagen molecule. Finally, fibrils coalesce into fibers, which gives collagen its tensile strength. In the gingiva, Type I collagen is the most prominent type, found in all layers of the gingival connective tissue. Type III collagen mainly underlies the gingival epithelium, and type IV collagen is associated with the gingival basement membranes and the blood vessels supplying the gingival mucosa.[4]
The ground substance found in gingival connective tissue is composed of proteoglycans and glycoproteins. Proteoglycans are large molecules made up of polysaccharides covalently bonded to proteins, and they function to regulate fluid flow and diffusion through the tissue matrix. Glycoproteins play a crucial role in maintaining the structural integrity of connective tissue. The key glycoprotein in gingival connective tissue is fibronectin, which orients fibroblasts to collagen and provides adequate attachment points for cellular adhesion to the connective tissue matrix.[5]
Embryology
Oral mucosal development
The oral mucosal epithelium primarily derives from the ectoderm (lips, cheeks, palate, gingivae, the floor of mouth) and endoderm (tongue). Early on in development during the first and second weeks of gestation, a single layer of epithelial cells lines the oral cavity. Subsequently, two cell layers between weeks 5 and 6, and by week 10, a multilayered epithelium is present. At this time, surface features of the oral mucosa, such as the papilla on the anterior two-thirds of the tongue and the palatal rugae, begin development. By approximately week 23 in utero, the oral epithelium has differentiated into stratified keratinized palatal and gingival epithelium, as well as stratified nonkeratinized epithelium, which lines the lips, cheeks, soft palate, and floor of the mouth.[6]
Dentogingival junction development
The development of the dentogingival junction is unique, as it involves the movement of teeth through the gingival epithelium and associated connective tissue, without causing epithelial breach or disruption. The tissues involved in the formation of this complex include the junctional epithelium (JE), sulcular epithelium, and underlying connective tissue. As a tooth comes in contact with the epithelial lining of the oral cavity, the connective tissue between the tooth and oral mucosa breakdown, and the basal layer of the oral epithelium begin to proliferate. Following this is the fusion between the reduced enamel epithelium and oral epithelium, with a breakdown of the central aspect of the epithelial surface. This development ensures no loss of epithelial continuity as the tip of the tooth descends into the oral mucosa. After the tooth enters the oral cavity, the cells of the enamel epithelium transform into JE cells, which act to maintain the junction between the enamel and epithelium via hemidesmosomes.[6]
Blood Supply and Lymphatics
The blood supply to gingiva consists of an intricate web of arteries that originate from the carotid artery. The entire buccal mucosa receives its supplied from tiny divisions of the buccal artery and posterior superior alveolar artery. The palate receives arterial supply from the greater palatine artery, a division of the descending palatine artery originating from the maxillary artery. The floor of the mouth and the mandibular lingual gingiva are both perfused by the sublingual and submental arteries. Finally, the labial gingiva overlying the mandible is perfused by small divisions from the inferior alveolar artery (incisive and mental artery). The labial gingiva overlying the maxilla is perfused by divisions from the anterior superior alveolar artery.[7][8][9]
Nerves
The innervation of the oral gingiva is unique because all the nerves supplying the gingival mucosa originate from the mandibular and maxillary branches of the trigeminal nerve (CN V).
The mandibular gingiva is innervated by various branches of the mandibular division of the trigeminal nerve: the lingual, inferior alveolar, and buccal nerves. After entering the infratemporal fossa and passing through the foramen ovale, the mandibular division divides into two trunks: the anterior trunk and posterior trunk.[8] The anterior trunk carries afferent sensory fibers that make up the buccal nerve, which innervates part of the lower oral gingiva. The posterior trunk splits into the lingual and inferior alveolar nerve. The lingual nerve then travels anteriorly into the oral cavity and provides sensory information to the lingual oral gingiva.[10] As the inferior alveolar nerve further descends into the mandible, it branches into the mylohyoid nerve and the mental nerve. While the mylohyoid nerve is responsible for motor innervation to the anterior belly of the digastric muscle and the mylohyoid muscle, the mental nerve provides sensory innervation to the lower gingival mucosa.[10]
The maxillary gingiva receives innervation from nerves originating from the maxillary division of the trigeminal nerve: the nasopalatine, greater palatine, and superior alveolar nerves. The greater and lesser palatine nerves pass through the palatine canal. The greater palatine nerve travels along the inferior surface of the hard palate and innervates the oral gingiva. The nasopalatine nerve, which is the longest of the nasal branches, travels through the incisive canal on the roof of the oral mucosa where it innervates the oral gingiva. Finally, the anterior, middle, and posterior superior alveolar nerves all contribute to buccal gingival innervation.[11]
Physiologic Variants
While the number of melanocytes in the oral epithelium is the same in all humans, there is a broad range of physiologic variation in oral gingival mucosa pigmentation. Thus, the variation in pigmentation is attributed to the activity of melanocytes in the basal cell layer of the oral epithelium. Therefore, it is the increased production melanin that is responsible for increased pigmentation. Oral gingiva pigmentation is more commonly seen in individuals with darker skin, regardless of their race or ethnicity, suggesting that genetic factors associated with melanogenesis are responsible for this physiologic pigmentation.[12]
Microscopic examination of melanocytes in the basal cell layer demonstrates increased melanin production, similar to the microscopic changes seen in pathologic pigmentation. However, it is essential to differentiate between pathologic and physiologic oral gingiva pigmentation. Diseases that are often confused with physiologic oral pigmentation include Addison disease, neurofibromatosis, oral mucosal melanoma, and drug-induced gingiva pigmentation.
Physiologic gingiva pigmentation affects males and females equally. The most common presentation is asymptomatic, solitary or multiple, well-defined dark macules in a symmetric distribution. Pigmentation gradually appears in the first two decades of life but often goes unnoticed. The intensity and amount of physiologic gingival pigmentation increase with age, which is attributable to the cumulative effects of inflammation and minor injury associated with aging.[12]
The presence of stippling characterizes the healthy gingiva in adults, often described as having an orange peel-like appearance. It is absent in infants and begins to appear at around age five years old. Stippling of gingiva is due to the rete pegs(prominences of the epithelial tissue) and depressions of the connective tissue.[13]
Surgical Considerations
Gingival recession describes the process by which the junctional epithelium of the oral gingiva recedes, leading to the pathologic exposure of the root of the tooth.[14] It is a clinical condition frequently seen in the general dental practice amongst patients with gingival disease. The gingival recession causes modifiable and nonmodifiable risk factors, as discussed in the following section.
Clinicians often have to decide whether or not to treat this condition surgically. Surgical correction of a gingival recession merits consideration when a patient is concerned about esthetics or tooth hypersensitivity, there is active gingival recession, or when the patient will undergo orthodontic treatment on a tooth with predisposing factors (inadequate keratinized mucosa, thin tissue-type). The two types of surgical procedures include root coverage or keratinized tissue augmentation. However, the benefits of surgical treatment lack substantial support in current literature relative to more conservative approaches such as management of the underlying etiological cause.[15]
Clinical Significance
Periodontal disease refers to a disease process that involves one of the four supporting structures surrounding a tooth, collectively known as the periodontium. These structures include the gingiva (gums), alveolar bone, cementum, and periodontal ligament. Gingivitis is a term used to describe inflammation of the junctional epithelial cells caused by bacteria and debris accumulation between the gum line and surface of the tooth. It is the mildest and most common form of periodontal disease, as it is present in up to 90% of the population. While gingivitis is potentially reversible with an improvement in oral hygiene, progression to periodontitis is irreversible and can have devastating effects. Periodontitis refers to a chronic, destructive, inflammatory disease state that occurs when bacteria penetrate deeper into the gingival tissue; this leads to a host immune response that attempts to defend against bacteria but causes periodontal destruction. Periodontitis leads to progressive alveolar bone loss and subsequent loss of the affected tooth.[16]
The etiology of periodontal disease can be attributed to several factors, which can be categorized as modifiable and non-modifiable risk factors. Modifiable risk factors include tobacco smoking, poor oral hygiene, diabetes, and pregnancy. One of the most important modifiable risk factors is tobacco smoking, as the risk for periodontal disease is 5 to 20 times higher in people who smoke compared to those who do not smoke.[17] Perhaps the most noteworthy risk factor is poor oral hygiene; as bacteria and plaque build-up in the tight spaces between the gingival mucosa and surface of the tooth, host inflammatory processes involved in defending against infection end up destroying bone and tissue. Diabetes contributes to gingivitis and periodontal disease by impairing wound healing, which can lead to gingival breakdown.[18] Elevated estrogen levels during pregnancy positively correlate with Porphyromonas gingivalis, a key microbe involved in the pathogenesis of gingivitis.[16]
Non-modifiable risk factors for gingivitis and associated periodontal disease include age and genetic disorders. The literature shows that the inflammatory response to plaque deposition in older individuals contains a significantly higher number of inflammatory cells than in younger individuals. Additionally, aging increases the risk of inadequate oral hygiene practices, further contributing to the increased risk of gingivitis and periodontal disease in the older population. Finally, genetic disorders such as Down syndrome, Ehlers-Danlos syndrome, and Crohn disease have proven to increase the risk of gingival disease.[16][19]
The most common initial presentation of early gingival disease is when an asymptomatic patient is discovered to have gingivitis during a routine dental visit. While symptoms are rare in early disease, the most common initial manifestation in early gingival disease is bleeding gums during brushing and flossing. Halitosis is another common symptom early in the disease. Patients at high risk who do not regularly visit the dentist often experience manifestations of late gingival disease, such as pain with chewing food, receding gums, and even loss of teeth. This deterioration is why routine dental screenings are vital in identifying early disease and initiating early treatment.[16]
Other Issues
The treatment of gingival disease and periodontitis involves a graded approach, always beginning with a professional dental cleaning. A critical part of the dental cleaning involves patient education by the dentist on the importance of maintaining healthy oral hygiene and instruction on how to improve their oral hygiene routine effectively. The most important aspect of treating gingival disease is reversing any modifiable risk factors. This intervention includes phase1 of the treatment, followed by phase 4, which is maintenance therapy.[16]
However, in cases that are refractory to non-pharmacological treatment, antibiotics should be considered. Initial antibiotic therapy should begin with topical chlorhexidine gluconate, which is an antimicrobial compound administered as a mouthwash. Treatment with systemic antibiotics is rare and should only be a consideration when other treatment methods fail. Systemic antimicrobial agents approved for use include tetracyclines, penicillins, macrolides, fluoroquinolones, and cephalosporins. Finally, gingival surgery to clean a periodontal pocket or correct gingival recession may be required if there has been significant anatomical disease destruction.